Check the below NCERT MCQ Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations with Answers Pdf free download. MCQ Questions for Class 10 Science with Answers were prepared based on the latest exam pattern. We have Provided Chemical Reactions and Equations Class 10 Science MCQs Questions with Answers to help students understand the concept very well.
Class 10 Science Chemistry Chapter 1 MCQ With Answers
You can refer to NCERT Solutions for Class 10 Science Chapter 1 Chemical reactions and equations to revise the concepts in the syllabus effectively and improve your chances of securing high marks in your board exams.
Chemistry Class 10 Chapter 1 MCQs on Chemical Reactions and Equations
Question 1.
Which of the following are exothermic processes?
(i) Reaction of water with quick lime
(ii) Dilution of an acid
(iii) Evaporation of water
(iv) Sublimation of camphor (crystals)
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (iii) and (iv)
Answer
Answer: (a) (i) and (ii)
Question 2.
A teacher gave two test tubes to the students, one containing water and the other containing sodium hydroxide. She asked them to identify the test tube containing sodium hydroxide solution. Which one of the following can be used for the identification?
(a) Blue litmus
(b) Red litmus
(c) Sodium carbonate solution
(d) Dilute hydrochloric acid
Answer
Answer: (b) Red litmus
Question 3.
Which of the following is not physical change?
(a) Boiling of water to give water vapour
(b) Melting of ice to give water
(c) Dissolution of salt in water
(d) Combustion of Liquefied Petroleum Gas (LPG)
Answer
Answer: (d) Combustion of Liquefied Petroleum Gas (LPG)
Question 4.
The following reaction is an example of a
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
(i) displacement reaction
(ii) combination reaction
(iii) redox reaction
(iv) neutralisation reaction
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i) and (iii)
(d) (iii) and (iv)
Answer
Answer: (c) (i) and (iii)
Question 5.
Which of the following is true for an unbalanced chemical equation?
(a) Number of atoms is equal on both sides of the equation
(b) Number of atoms is less on the left side of the equation
(c) Number of atoms is more on the right side of the equation
(d) Both (b) and (c).
Answer
Answer: (d) Both (b) and (c).
Question 6.
Which option denotes a double displacement reaction?
(a) A + B + C
(b) A + B → C
(c) AC + BD → AD + BC
(d) AC + B → AB + C
Answer
Answer: (c) AC + BD → AD + BC
Question 7.
Which among the following is (are) double displacement reaction(s)?
(t) Pb + CuCl → PbCl2 + Cu
(ii) Na2SO4 + BaCl2 → BaSO4 + 2NaCl
(iii) C + O2 → CO2
(iv) CH4 + 2O2 → CO2 + 2H2O
(a) (i) and (iv)
(b) (ii) only
(c) (i) and (ii)
(d) (iii) and (iv)
Answer
Answer: (b) (ii) only
Question 8.
The following reaction is used for the preparation of oxygen gas in the laboratory:
![]()
Which of the following statement about the reaction is correct?
(a) It is a decomposition reaction and endothermic in nature.
(b) It is a combination reaction.
(c) It is a decomposition reaction and accompanied by release of heat.
(d) It is a photochemical decomposition reaction and exothermic in nature.
Answer
Answer: (a) It is a decomposition reaction and endothermic in nature.
Question 9.
Which of the following is Not True with respect to the neutralisation reaction?
(a) Salt is formed.
(b) Reaction occurs between an acid and a base.
(c) Reactive element displaces less reactive element.
(d) Reactants are in gaseous state.
Answer
Answer: (d) Reactants are in gaseous state.
Question 10.
Electrolysis of water is a decomposition reaction. The mole ratio of hydrogen and oxygen gases liberated during electrolysis of water is
(a) 1 : 1
(b) 2 : 1
(c) 4 : 1
(d) 1 : 2
Answer
Answer: (b) 2 : 1
Question 11.
Combustion reactions are always
(a) Exothermic
(b) Endothermic
(c) Sometimes exothermic
(d) Both (a) and (b).
Answer
Answer: (a) Exothermic
Question 12.
Barium chloride on reacting with ammonium sulphate forms barium sulphate and ammonium chloride. Which of the following correctly represents the type of the reaction involved?
(i) Displacement reaction
(ii) Precipitation reaction
(iii) Combination reaction
(iv) Double displacement reaction
(a) (i) only
(b) (ii) only
(c) (iv) only
(d) (ii) and (iv)
Answer
Answer: (d) (ii) and (iv)
Question 13.
Which one of the following processes involve chemical reactions?
(a) Storing of oxygen gas under pressure in a gas cylinder
(b) Liquefaction of air
(c) Keeping petrol in a China dish in the open
(d) Heating copper wire in presence of air at high temperature
Answer
Answer: (d) Heating copper wire in presence of air at high temperature
Question 14.
In the double displacement reaction between aqueous potassium iodide and aqueous lead nitrate, a yellow precipitate of lead iodide is formed. While performing the activity if lead nitrate is not available, which of the following can be used in place of lead nitrate?
(a) Lead sulphate (insoluble)
(b) Lead acetate
(c) Ammonium nitrate
(d) Potassium sulphate
Answer
Answer: (b) Lead acetate
Question 15.
In which of the following chemical equations, the abbreviations represent the correct states of the reactants and products involved at reaction temperature?
(a) 2H2(l) + O2(l) → 2H2O(g)
(b) 2H2(g) + O2(l) → 2H2O(l)
(c) 2H2(g) + O2(g) → 2H2O(l)
(d) 2H2(g) + O2(g) → 2H2O(g)
Answer
Answer: (d) 2H2(g) + O2(g) → 2H2O(g)
Fill in the blanks
1. ………….. is burnt in air to form magnesium oxide.
Answer
Answer: Magnesium
2. Respiration is an ………….. reaction.
Answer
Answer: exothermic
3. Zinc and lead are more reactive elements than …………..
Answer
Answer: copper
4. Any reaction that produces a ………….. is called a precipitation reaction.
Answer
Answer: Precipitate
5. Oxidation is the gain of ………….. or loss of …………..
Answer
Answer: oxygen, hydrogen
6. ………….. are added to foods containing fats and oil.
Answer
Answer: Antioxidants
7. In rusting of iron, reddish brown coating formed on iron is …………..
Answer
Answer: Fe2O3
8. ………….. can be retarded by storing foods away from light.
Answer
Answer: Rancidity
9. The oxidation and reduction reaction are also called ………….. reactions.
Answer
Answer: redox
10. Magnesium is able to displace copper from copper sulphate solution because magnesium is ………….. reactive than copper.
Answer
Answer: more
Match the following columns
1. For the given reaction, match column I with column II and mark the correct option from the codes given below.
Fe2O3 + xCO → yFe + xCO2
| Column I | Column II |
| (a) Oxidising agent | (p) 2 |
| (b) Reducing agent | (q) 3 |
| (c) x | (r) Fe2O3 |
| (d) y | (s) CO |
Answer
Answer:
| Column I | Column II |
| (a) Oxidising agent | (r) Fe2O3 |
| (b) Reducing agent | (s) CO |
| (c) x | (q) 3 |
| (d) y | (p) 2 |
2.
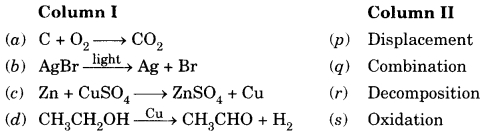
Answer
Answer:
(a) → (q)
(b) → (r)
(c) → (p)
(d) → (s)
3.
| Symbols | Meaning |
| (a) → | (p) Aqueous |
| (b) (g) | (q) Yield or gives |
| (c) Δ | (r) Heat |
| (d) (aq) | (s) Liberation of gas |
Answer
Answer:
| Symbols | Meaning |
| (a) → | (q) Yield or gives |
| (b) (g) | (s) Liberation of gas |
| (c) Δ | (r) Heat |
| (d) (aq) | (p) Aqueous |
4.
| Column I | Column II |
| (a) Combination reaction | (p) Synthesis |
| (b) Oxidation of iron | (q) Splitting-up of reactants |
| (c) Displacement reaction | (r) Combustion |
| (d) Decomposition | (s) Substitution |
Answer
Answer:
| Column I | Column II |
| (a) Combination reaction | (p) Synthesis |
| (b) Oxidation of iron | (r) Combustion |
| (c) Displacement reaction | (s) Substitution |
| (d) Decomposition | (q) Splitting-up of reactants |
Crossword
1. Down:
1. Phenomenon in which iron vessels get damaged on adding copper sulphate solution (12)
3. Phenomenon in which food material starts to smell badly on keeping (9)
Across:
2. A reaction between acids and bases (14)
4. A reaction in which one of the products becomes insoluble (13)
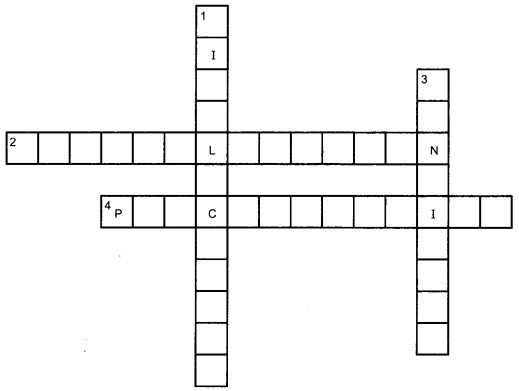
Answer
Answer:
Down:
1. Displacement
3. Rancidity
Across:
2. Neutralisation
4. Precipitation
We hope the given NCERT MCQ Questions for Class 10 Science Chapter 1 Chemical Reactions and Equations with Answers Pdf free download will help you. If you have any queries regarding Chemical Reactions and Equations CBSE Class 10 Science MCQs Multiple Choice Questions with Answers, drop a comment below and we will get back to you soon.
Class 10 Science Chemistry MCQ With Answers
- Chemical Reactions and Equations Class 10 MCQ
- Acids, Bases and Salts Class 10 MCQ
- Metals and Non-metals Class 10 MCQ
- Carbon and Its Compounds Class 10 MCQ
- Periodic Classification of Elements Class 10 MCQ