Check the below NCERT MCQ Questions for Class 10 Science Chapter 3 Metals and Non-metals with Answers Pdf free download. MCQ Questions for Class 10 Science with Answers were prepared based on the latest exam pattern. We have Provided Metals and Non-metals Class 10 Science MCQs Questions with Answers to help students understand the concept very well.
Class 10 Science Chemistry Chapter 3 MCQ With Answers
You can refer to NCERT Solutions for Class 10 Science Chapter 3 Metals and Non-metals to revise the concepts in the syllabus effectively and improve your chances of securing high marks in your board exams.
Chemistry Class 10 Chapter 3 MCQs on Metals and Non-metals
Question 1.
The ability of metals to be drawn into thin wires is known as
(a) ductility
(b) malleability
(c) sonority
(d) conductivity
Answer
Answer: (a) ductility
Question 2.
Aluminium is used for making cooking utensils. Which of the following properties of aluminium are responsible for the same?
(i) Good thermal conductivity
(ii) Good electrical conductivity
(iii) Ductility
(iv) High melting point
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (i) and (iv)
Answer
Answer: (d) (i) and (iv)
Question 3.
Due to its semiconductor properties the non-metal used in computer, T.V. etc. is
(a) Carbon
(b) Silicon
(c) Bromine
(d) Fluorine
Answer
Answer: (b) Silicon
Question 4.
What happens when calcium is treated with water?
(i) It does not react with water.
(ii) It reacts violently with water.
(iii) It reacts less violently with water.
(iv) Bubbles of hydrogen gas formed stick to the surface of calcium.
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i) and (ii)
(d) (iii) and (iv)
Answer
Answer: (d) (iii) and (iv)
Question 5.
Generally metals react with acids to give salt and hydrogen gas. Which of the following acids does not give hydrogen gas on reacting with metals (except Mn and Mg)?
(a) H2SO4
(b) HCl
(c) HNO3
(d) All of these
Answer
Answer: (c) HNO3
Question 6.
Which of the following metals are obtained by electrolysis of their chlorides in molten state?
(i) Na
(ii) Ca
(iii) Fe
(iv) Cu
(a) (i) and (iv)
(b) (iii) and (iv)
(c) (i) and (iii)
(d) (i) and (ii)
Answer
Answer: (d) (i) and (ii)
Question 7.
An alloy reacted with dilute hydrochloric acid to produce a gas which ‘pops’ a lighted splint. The residue reacted with dilute nitric acid to form a blue solution. Which one of the following pairs of metals is present in the alloy?
(a) Copper and lead
(b) Lead and magnesium
(c) Copper and magnesium
(d) Lead and zinc
Answer
Answer: (c) Copper and magnesium
Question 8.
Which of the following metals exist in their native state in nature?
(i) Cu
(ii) Au
(iii) Zn
(iv) Ag
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (iii) and (iv)
Answer
Answer: (c) (ii) and (iv)
Question 9.
Metals are refined by using different methods. Which of the following metals are refined by electrolytic refining?
(i) Au
(ii) Cu
(iii) Na
(iv) K
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (iii)and (iv)
Answer
Answer: (a) (i) and (ii)
Question 10.
Metal M occurs in the Earth’s crust as its oxide M2O3. An alloy of this metal is used in making aircrafts. The ore of the metal M is
(a) magnetite
(b) haematite
(c) bauxite
(d) pyrolusite
Answer
Answer: (c) bauxite
Question 11.
Which one of the following four metals would be displaced from the solution of its salts by other three metals?
(a) Mg
(b) Ag
(c) Zn
(d) Cu
Answer
Answer: (b) Ag
Question 12.
2 mL each of concentrated HCl, HNO3 and a mixture of concentrated HCl and concentrated HNO3 in the ratio of 3 : 1 were taken in test tubes labelled as A, B and C. A small piece of metal was put in each test tube. No change occurred in test tubes A and B but the metal got dissolved in test tube C respectively. The metal could be
(a) Al
(b) Au
(c) Cu
(d) Pt
Answer
Answer: (b) Au
Question 13.
Which one of the following figures correctly describes the process of electrolytic refining?
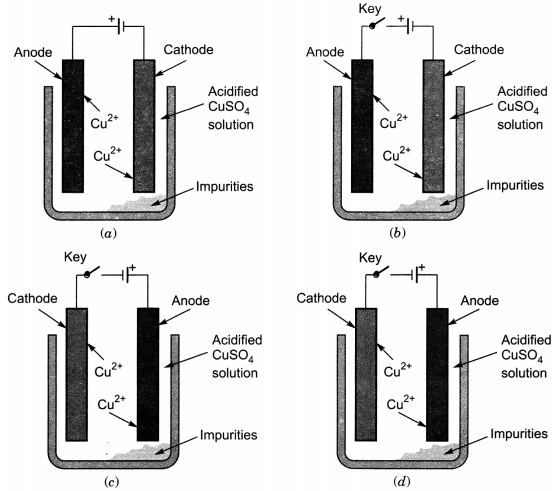
Answer
Answer: (c)
Question 14.
An element A is soft and can be cut with a knife. This is very reactive to air and cannot be kept in open. It reacts vigorously with water. Identify the element from the following:
(a) Mg
(b) Na
(c) P
(d) Ca
Answer
Answer: (b) Na
Question 15.
Alloys are homogeneous mixtures of a metal with a metal or non-metal. Which among the following alloys contain non-metal as one of its constituents?
(a) Brass
(b) Bronze
(c) Amalgam
(d) Steel
Answer
Answer: (d) Steel
Question 16.
Reaction between X and Y, forms compound Z. X loses electron and Y gains electron. Which of the following properties is not shown by Z?
(a) Has high melting point
(b) Has low melting point
(c) Conducts electricity in molten state
(d) Occurs as solid
Answer
Answer: (b) Has low melting point
Question 17.
The electronic configurations of three elements X, Y and Z are X – 2, 8; Y – 2, 8, 7 and Z – 2, 8, 2. Which of the following is correct?
(a) X is a metal.
(b) Y is a metal.
(c) Z is a non-metal.
(d) Y is a non-metal and Z is a metal.
Answer
Answer: (d) Y is a non-metal and Z is a metal.
Question 18.
Generally, non-metals are not conductors of electricity. Which of the following is a good conductor of electricity?
(a) Diamond
(b) Graphite
(c) Sulphur
(d) Fullerene
Answer
Answer: (b) Graphite
Question 19.
Which of the following can undergo a chemical reaction?
(a) MgSO4 + Fe
(b) ZnSO2 + Fe
(c) MgSO2 + Pb
(d) CuSO2 + Fe
Answer
Answer: (d) CuSO2 + Fe
Question 20.
The atomic numbers of four elements A, B, C and D are 6, 8, 10 and 12 respectively. The two elements which can react to form ionic bonds (or ionic compound) are:
(a) A and D
(b) B and C
(c) A and C
(d) B and D
Answer
Answer: (d) B and D
Question 21.
The atomic number of an element X is 19. The number of electrons in its ion X+ will be:
(a) 18
(b) 19
(c) 20
(d) 21
Answer
Answer: (a) 18
Question 22.
The atomic number of an element Y is 17. The number of electrons in its ion Y– will be:
(a) 17
(b) 18
(c) 19
(d) 20
Answer
Answer: (b) 18
Question 23.
Which of the following is an iron ore?
(a) Cinnabar
(b) Calamine
(c) Haematite
(d) Rock salt
Answer
Answer: (c) Haematite
Question 24.
The metal which can be extracted from the bauxite ore is:
(a) Na
(b) Mn
(c) Al
(d) Hg
Answer
Answer: (c) Al
Question 25.
In stainless steel alloy, iron metal is mixed with:
(a) Cu and Cr
(b) Cr and Ni
(c) Cr and Sn
(d) Cu and Ni
Answer
Answer: (b) Cr and Ni
Question 26.
Which of the following is an ore of mercury metal?
(a) Rock salt
(b) Cinnabar
(c) Calamine
(d) Haematite
Answer
Answer: (b) Cinnabar
Question 27.
Which of the following pair of metals exist in their native state in nature?
(a) Ag and Hg
(b) Ag and Zn
(c) Au and Hg
(d) Au and Ag
Answer
Answer: (d) Au and Ag
Question 28.
Which of the following alloys contains a non-metal as one of the constituents?
(a) Brass
(b) Amalgam
(c) Steel
(d) Bronze
Answer
Answer: (c) Steel
Question 29.
The metal which is always present in an amalgam is:
(a) iron
(b) aluminium
(c) mercury
(d) magnes
Answer
Answer: (c) mercury
Question 30.
Rock salt is an ore of one of the following metals. This metal is:
(a) Mn
(b) Na
(c) Fe
(d) Cu
Answer
Answer: (b) Na
Fill in the blanks
1. The process in which the concentrated ore is heated below its melting point in the absence or limited supply of air is called …………….
Answer
Answer: Calcination
2. Metal have ……………. melting and boiling points.
Answer
Answer: high
3. ……………. displaces copper from a solution of copper (II) sulphate.
Answer
Answer: Zinc
4. Removal of impurities from a metal by chemical method is called …………….
Answer
Answer: leaching
5. Oxides of non-metals when dissolved in water generally give ……………. solutions.
Answer
Answer: acidic
6. A sulphide ore is concentrated by …………….
Answer
Answer: froth flotation process
7. The chlorides of non-metals are …………….
Answer
Answer: covalent
8. Non-metals are ……………. in character.
Answer
Answer: electronegative
9. The impurities in the ores are removed by adding …………….
Answer
Answer: flux
10. The rocky material found along with ores is known as …………….
Answer
Answer: gangue
Match the following columns
Four substances have the following electrical properties. What are these four substances?
| Substance | Electrical property |
| W | Does not conduct under any conditions |
| X | Conducts only in aqueous solution |
| Y | Conducts in both the molten and solid |
| Z | Conducts in both the molten and aqueous |
1.
| Column I | Column II |
| (a) W | (p) NaCl |
| (b) X | (q) Pb |
| (c) Y | (r) S |
| (d) Z | (s) HCl |
Answer
Answer:
| Column I | Column II |
| (a) W | (r) S |
| (b) X | (s) HCl |
| (c) Y | (q) Pb |
| (d) Z | (p) NaCl |
2.
| Column I | Column II |
| (a) Hydraulic washing | (p) Difference in solubility of gangue and ore particles in a specific substance |
| (b) Magnetic separation | (q) Difference in wetting property of gangue and ore particles. |
| (c) Froth flotation | (r) Difference in gravities of ore and gangue particles |
| (d) Leaching | (s) Difference in magnetic properties of ore and gangue particles |
Answer
Answer:
| Column I | Column II |
| (a) Hydraulic washing | (r) Difference in gravities of ore and gangue particles |
| (b) Magnetic separation | (s) Difference in magnetic properties of ore and gangue particles |
| (c) Froth flotation | (q) Difference in wetting property of gangue and ore particles. |
| (d) Leaching | (p) Difference in solubility of gangue and ore particles in a specific substance |
3.
| Column I | Column II |
| (a) Haematite | (p) Carbonate |
| (b) Camallite | (q) Oxide |
| (c) Dolomite | (r) Sulphate |
| (d) Anglesite | (s) Chloride |
Answer
Answer:
| Column I | Column II |
| (a) Haematite | (q) Oxide |
| (b) Camallite | (s) Chloride |
| (c) Dolomite | (p) Carbonate |
| (d) Anglesite | (r) Sulphate |
Complete the crossword given below.
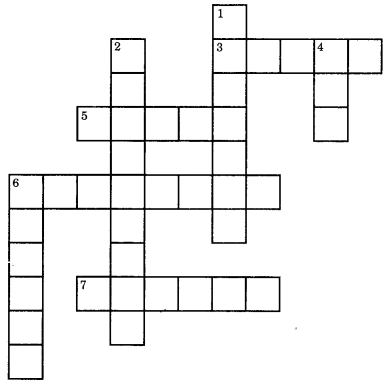
Across
3. A homogeneous mixture of two or more metals
5. An alloy of copper and zinc
6. Property of metal due to which metal produce a sound on striking a hard surface
7. Lustrous non-metal
Down
1. Metal which melt if you keep them on your palm
2. A phenomenon by which a metal get corroded when they are exposed to moist air
4. Substance from which metal can be extracted by metallurgy
6. Metal which can be cut with knife
Answer
Answer:
Across:
3. Alloy
5. Brass
6. Sonorous
7. Iodine
Down:
1. Caesium
2. Corrosion
4. Ore
6. Sodium
We hope the given NCERT MCQ Questions for Class 10 Science Chapter 3 Metals and Non-metals with Answers Pdf free download will help you. If you have any queries regarding Metals and Non-metals CBSE Class 10 Science MCQs Multiple Choice Questions with Answers, drop a comment below and we will get back to you soon.
Class 10 Science Chemistry MCQ With Answers
- Chemical Reactions and Equations Class 10 MCQ
- Acids, Bases and Salts Class 10 MCQ
- Metals and Non-metals Class 10 MCQ
- Carbon and Its Compounds Class 10 MCQ
- Periodic Classification of Elements Class 10 MCQ