Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Hydrogen Bonding
Hydrogen bonding is one of the most important natural phenomena occurring in chemical and biological sciences. These interactions play a major role in the structure of proteins and DNA. When a hydrogen atom (H) is covalently bonded to a highly electronegative atom such as flourine (F) or oxygen (O) or nitrogen (N), the bond is polarized.
Due to this effect, the polarized hydrogen atom is able to form a weak electrostatic interaction with another electronegative atom present in the vicinity. This interaction is called as a hydrogen bond (20-50 kJ mol-1) and is denoted by dotted lines (…).
It is weaker than covalent bond (>100 kJ mol-1) but stronger than the van der Waals interaction (< 20 kJ mol-1). Hydrogen bond has profound effct on various physical properties including vapour pressure (H2O and H2S), boiling point, miscibility of liquids (H2O and C2H5OH), surface tension, densities, viscosity, heat of vapourization and fusion, etc. Hydrogen bonds can occur within a molecule (intramolecular hydrogen bonding) and between two molecules of the same type or different type (intermolecular hydrogen bonding).
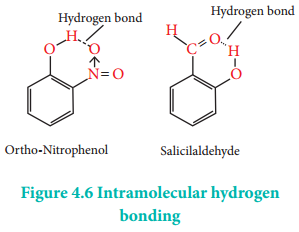
Intramolecular Hydrogen Bond
Intramolecular hydrogen bonds are those which occur within a single molecule.
Intermolecular Hydrogen Bond
Intermolecular hydrogen bonds occur between two separate molecules. They can occur between any numbers of like or unlike molecules as long as hydrogen donors and acceptors are present in positions which enable the hydrogen bonding interactions. For example, intermolecular hydrogen bonds can occur between ammonia molecule themselves or between water molecules themselves or between ammonia and water.
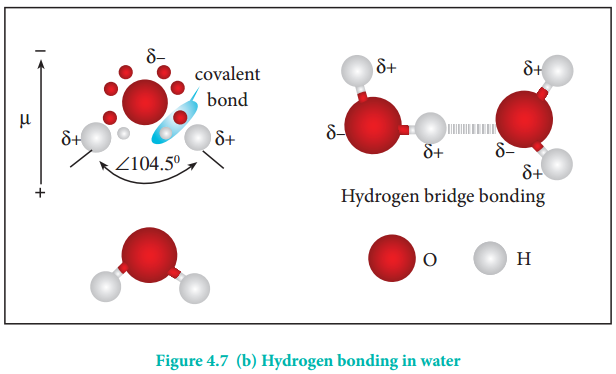
Water molecules form strong hydrogen bonds with one another. For example, each water molecule is linked to four others through hydrogen bonds. The shorter distances (100 pm) correspond to covalent bonds (solid lines), and the longer distances (180 pm) correspond to hydrogen bonds (dotted lines).
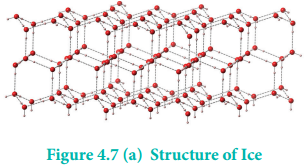
In ice, each atom is surrounded tetrahedrally by four water molecules through hydrogen bonds. That is, the presence of two hydrogen atoms and two lone pairs of electron on oxygen atoms in each water molecule allows formation of a three-dimensional structure. This arrangement creates an open structure, which accounts for the lower density of ice compared with water at 0°C. While in liquid water, unlike ice where hydrogen bonding occurs over a long-range, the strong hydrogen bonding prevails only in a short range and therefore the denser packing.
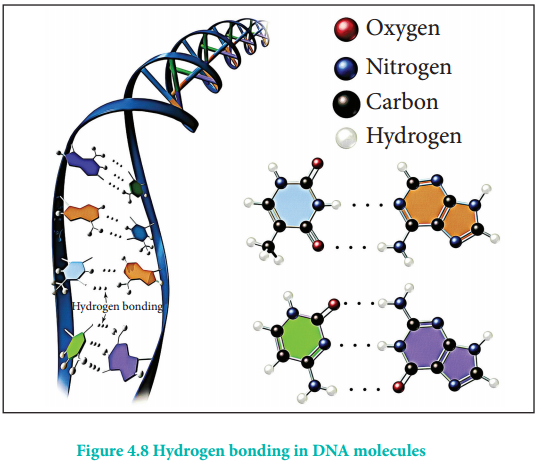
Hydrogen bond occurs not only in simple molecules but also in complex biomolecules such as proteins, and they are crucial for biological processes. For example, hydrogen bonds play an important role in the structure of deoxyribonucleic acid (DNA), since they hold together the two helical nucleic acid chains (strands).