slot gacor malam ini slot gacor malam ini Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world. Biomolecules of Vitamins and Their Functions Vitamins are small organic compounds that cannot be synthesised by our body but are essential for certain functions. Hence, they must be obtained through diet. The requirements … [Read more...] about Biomolecules of Vitamins and Their Functions
Chemistry
Food Additives of Chemistry In Everday Life
https://api-wa.sumedangkab.go.id/hgacor/ Daftar Slot Gacor link slot bca gacor https://api-wa.sumedangkab.go.id/hgacor/ Daftar Slot Gacor link slot bca gacor https://api-wa.sumedangkab.go.id/hgacor/ Daftar Slot Gacor link slot bca gacor daftar slot gacor ayo188 bpjs88 mamibet66 tps4d Daftar Slot Gacor situs slot malam ini Event Parlay Sultan situs taruhan Rtp Slot … [Read more...] about Food Additives of Chemistry In Everday Life
Biological Importance of Magnesium and Calcium
Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world. Biological Importance of Magnesium and Calcium Magnesium and calcium also plays a vital role in biological functions. A typical adult human body contains about 25g of magnesium and 1200g of calcium. Magnesium plays an important role in many biochemical reactions … [Read more...] about Biological Importance of Magnesium and Calcium
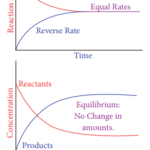
Law of Mass Action
Law of Mass Action In 1864 two Norwegian chemists namely Maximilian Guldberg and Peter Waage formulated the law of mass action, based on the experimental studies of many reversible reactions. The law states that, “At any instant, the rate of a chemical reaction at a given temperature is directly proportional to the product of the active masses of the reactants at that … [Read more...] about Law of Mass Action
Homogeneous and Hetrogeneous Equilibrium
Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world. Homogeneous and Hetrogeneous Equilibrium In a homogeneous equilibrium, all the reactants and products are in the same phase. For example H2(g) + I2(g) ⇄ 2HI(g) In the above equilibrium, H2, I2 and HI are in the gaseous state. Similarly, for the following … [Read more...] about Homogeneous and Hetrogeneous Equilibrium