Check the below NCERT MCQ Questions for Class 9 Science Chapter 2 Is Matter Around Us Pure with Answers Pdf free download. MCQ Questions for Class 9 Science with Answers were prepared based on the latest exam pattern. We have Provided Is Matter Around Us Class 9 Science MCQs Questions with Answers to help students understand the concept very well.
Class 9 Science Chemistry Chapter 2 MCQ With Answers
You can refer to NCERT Solutions for Class 9 Science Chapter 2 Is Matter Around Us Pure to revise the concepts in the syllabus effectively and improve your chances of securing high marks in your board exams.
Chemistry Class 9 Chapter 2 MCQs on Is Matter Around Us Pure
Question 1.
Which of the following statements are true for pure substances?
(i) Pure substances contain only one kind of particles
(ii) Pure substances may be compound or mixtures
(iii) Pure substances have the same composition throughout
(iv) Pure substances can be exemplified by all elements other than nickel
(a) (i) and (ii)
(b) (i) and (iii)
(c) (iii) and (iv)
(d) (ii) and (iii)
Answer
Answer: (b) (i) and (iii)
Question 2.
Rusting of an article made up of iron is called
(a) corrosion and it is a physical as well as chemical change
(b) dissolution and it is a physical change
(c) corrosion and it is a chemical change
(d) dissolution and it is a chemical change
Answer
Answer: (c) corrosion and it is a chemical change
Question 3.
A mixture of sulphur and carbon disulphide is
(a) heterogeneous and shows Tyndall effect
(b) homogeneous and shows Tyndall effect
(c) heterogeneous and does not show Tyndall effect
(d) homogeneous and does not show Tyndall effect
Answer
Answer: (d) homogeneous and does not show Tyndall effect
Question 4.
Tincture of iodine has antiseptic properties. This solution is made by dissolving
(a) iodine in potassium iodide
(b) iodine in vaseline
(c) iodine in water
(d) iodine in alcohol
Answer
Answer: (d) iodine in alcohol
Question 5.
Which of the following are homogeneous in nature?
(i) ice
(ii) wood
(iii) soil
(iv) air
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (i) and (iv)
(d) (iii) and (iv)
Answer
Answer: (c) (i) and (iv)
Question 6.
Blood and sea water are
(a) both mixtures
(b) both are compounds
(c) blood is a mixture whereas sea water is a compound
(d) blood is a compound and sea water is a mixture
Answer
Answer: (a) both mixtures
Question 7.
Sol and gel are examples of
(a) Solid-solid colloids
(b) Sol is a solid-liquid colloid and gel is liquid solid colloid
(c) Sol is solid-solid colloid and gel is solid-liquid colloid
(d) Sol is a liquid-solid colloid and gel is a solid-liquid colloid
Answer
Answer: (b) Sol is a solid-liquid colloid and gel is liquid solid colloid
Question 8.
In a water-sugar solution
(a) water is solute and sugar is solvent
(b) water is solvent and sugar is solute
(c) water is solute and water is also solvent
(d) none of these
Answer
Answer: (b) water is solvent and sugar is solute
Question 9.
Boron and carbon are
(a) metalloids
(b) metalloid and non-metal respectively
(c) metal
(d) non-metal and metalloid respectively
Answer
Answer: (b) metalloid and non-metal respectively
Question 10.
Which of the following are physical changes?
(i) Melting of iron metal
(ii) Rusting of iron
(iii) Bending of an iron rod
(iv) Drawing a wire of iron metal
(a) (i), (ii) and (iii)
(b) (i), (ii) and (iv)
(c) (i), (iii) and (iv)
(d) (ii), (iii) and (iv)
Answer
Answer: (c) (i), (iii) and (iv)
Question 11.
Which of the following are chemical changes?
(i) Decaying of wood
(ii) Burning of wood
(iii) Sawing of wood
(iv) Hammering of a nail into a piece of wood
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (i) and (iv)
Answer
Answer: (a) (i) and (ii)
Question 12.
Two substances, A and B were made to react to reaction a third substance, A2B according to the following
2 A + B → A2B
Which of the following statements concerning this reaction are incorrect?
(i) The product A2B shows the properties of substances A and B
(ii) The product will always have a fixed composition
(iii) The product so formed cannot be classified as a compound
(iv) The product so formed is an element
(a) (i), (ii) and (iii)
(b) (ii), (iii) and (iv)
(c) (i), (iii) and (iv)
(d) (ii), (iii) and (iv)
Answer
Answer: (c) (i), (iii) and (iv)
Question 13.
Two chemical species X and Y combine together to form a product P which contains both X and Y
X + Y → P
X and Y cannot be broken down into simpler substances by simple chemical reactions. Which of the following concerning the species X, Y and P are correct?
(i) P is a compound
(ii) X and Y are compounds
(iii) X and Y are elements
(iv) P has a fixed composition
(a) (i), (ii) and (iii)
(b) (i), (ii) and (iv)
(c) (ii), (iii) and (iv)
(d) (i), (iii) and (iv)
Answer
Answer: (d) (i), (iii) and (iv)
Question 14.
Which of the following methods would you use to separate cream from milk?
(a) Fractional distillation
(b) Distillation
(c) Centrifugation
(d) Filtration
Answer
Answer: (c) Centrifugation
Question 15.
Cooking of food and digestion of food:
(a) are both physical processes
(b) are both chemical processes
(c) cooking is physical whereas digestion is chemical
(d) cooking is chemical whereas digestion is physical
Answer
Answer: (b) are both chemical processes
Question 16.
Mercury and bromine are both
(a) liquid at room temperature
(b) solid at room temperature
(c) gases at room temperature
(d) both (a) and (b)
Answer
Answer: (a) liquid at room temperature
Classify the substances given in figure into elements and compounds.
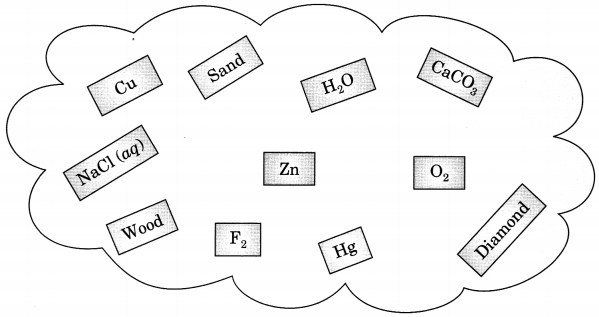
Answer
Answer:
Elements:
Cu
Zn
O2
Diamond (carbon)
Hg
Compounds:
CaCO3
H2O
Sand
Nacl(aq)
wood
Complete the crossword given below:
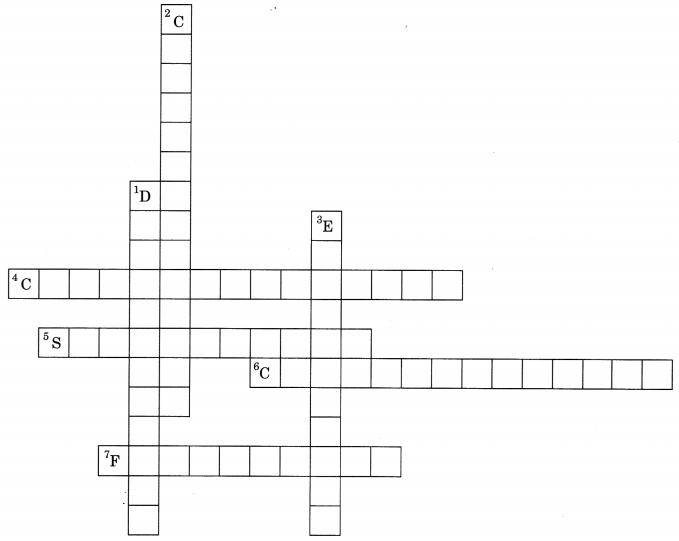
Down:
1. Process of separating two miscible liquids.
2. Technique of separating cream from milk.
3. Method of separating salt from sea water.
Across:
4. Method of purifying solids.
5. Process of changing a solid directly into gaseous state.
6. Technique of separating two or more colours of a dye.
7. A method of separation of insoluble solid from a mixture.
Answer
Answer:
Down:
1. Distillation
2. Centrifugation
3. Evaporation
Across:
4. Crystallisation
5. Sublimation
6. Chromatography
7. Filtration
Fill in the blanks:
1. A colloid is a ………….. mixture and its components can be separated by the technique known as ……………
Answer
Answer: heterogeneous, centrifugation
2. Ice, water and water vapour look different and display different …………. properties but they are ………… the same.
Answer
Answer: physical, chemically
3. A mixture of chloroform and water taken in a separating funnel is mixed and left undisturbed for sometime. The upper layer in the separating funnel will be of ………….. and the lower layer will be that of …………..
Answer
Answer: water, chloroform (hint-density of water is less than that of chloroform)
4. A mixture of two or more miscible liquids, for which the difference in the boiling points is less than 25 K can be separated by the process called ………….
Answer
Answer: fractional distillation
5. When light is passed through water containing a few drops of milk, it shows a bluish tinge. This is due to the …………… of light by milk and the phenomenon is called ………….. This indicates that milk is a …………… solution.
Answer
Answer: scattering, Tyndall effect, colloidal.
We hope the given NCERT MCQ Questions for Class 9 Science Chapter 2 Is Matter Around Us with Answers Pdf free download will help you. If you have any queries regarding Is Matter Around Us CBSE Class 9 Science MCQs Multiple Choice Questions with Answers, drop a comment below and we will get back to you soon.
Class 9 Science Chemistry MCQ With Answers
- Matter in Our Surroundings Class 9 MCQ
- Is Matter Around Us Pure Class 9 MCQ
- Atoms and Molecules Class 9 MCQ
- Structure of the Atom Class 9 MCQ