Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Introduction of Thermodynamics
The term ‘Thermodynamics’ means flow of heat and is derived from the Greek ‘Thermos’ (heat) and ‘dynamics’ (flow), In our daily life, we come across many useful reactions such as burning of fuel to produce heat energy, flow of electrons through circuit to produce electrical energy, metabolic reactions to produce the necessary energy for biological functions and so on.
Thermodynamics, the study of the transformation of energy, explains all such processes quantitatively and allows us to make useful predictions. In the 19th century, scientists tried to understand the underlying principles of steam engine which were already in operation, in order to improve their efficiency.
The basic problem of the investigation was the transformation of heat into mechanical work. However, over time, the laws of thermodynamics were developed and helped to understand the process of steam engine. These laws have been used to deduce powerful mathematical relationships applicable to a broad range of processes.
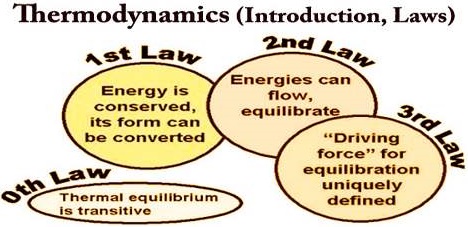
Thermodynamics evaluates the macroscopic properties (heat, work) and their inter relationships. It deals with properties of systems in equilibrium and is independent of any theories or properties of the individual molecules which constitute the system. The principles of thermodynamics are based on three laws of thermodynamics.
The first two laws (First and second law) summarise the actual experience of inter conversion of different forms of energy. The third law deals with the calculation of entropy and the unattainability of absolute zero Kelvin. Thermodynamics carries high practical values but bears certain limitations. It is independent of atomic and molecular structure and reaction mechanism.
The laws can be used to predict whether a particular reaction is feasible or not under a given set of conditions, but they cannot give the rate at which the reaction takes place. In other words, thermodynamics deals with equilibrium conditions quantitatively, but does not take into account the kinetic approach to the equilibrium state.
Thermodynamics is the study of the energy, principally heat energy, that accompanies chemical or physical changes. Others absorb heat energy and are called endothermic reactions, and they have a positive enthalpy change. But thermodynamics is concerned with more than just heat energy.
Thermodynamics is the study of the relations between heat, work, temperature, and energy. The laws of thermodynamics describe how the energy in a system changes and whether the system can perform useful work on its surroundings.
Basic Principles of Thermodynamics. A thermodynamical system is an arbitrarily but suitable chosen region of the space where certain phenomena are investigated. The system is enclosed by its surroundings. The system and its surroundings may be in equilibrium or may be interactions between them.
Thermodynamics is the science of the relationship between heat, work and the properties of substances. While the Zeroth Law provides the basis of measurement of Temperature, the First and Second Laws serve to define the two properties, Energy and Entropy, and deal with the conservation and degradation of energy.
Thermodynamics is a very important branch of both physics and chemistry. It deals with the study of energy, the conversion of energy between different forms and the ability of energy to do work.
Here are some more applications of thermodynamics: Sweating in a crowded room: In a crowded room, everybody (every person) starts sweating. The body starts cooling down by transferring the body heat to the sweat. Sweat evaporates adding heat to the room.
Thermodynamics is that part of science which is concerned with the conditions that material systems may assume and the changes in conditions that may occur either spontaneously or as a result of interactions between systems. The word “thermodynamics” was derived from the Greek words thermé (heat) and dynamics (force).
One of the most important things we can do with heat is to use it to do work for us. A heat engine does exactly this it makes use of the properties of thermodynamics to transform heat into work. Gasoline and diesel engines, jet engines, and steam turbines that generate electricity are all examples of heat engines.
The most common practical application of the First Law is the heat engine. Heat engines convert thermal energy into mechanical energy and vice versa. Most heat engines fall into the category of open systems.