Students can access the CBSE Sample Papers for Class 10 Science with Solutions and marking scheme Term 2 Set 3 will help students in understanding the difficulty level of the exam.
CBSE Sample Papers for Class 10 Science Term 2 Set 3 with Solutions
Time : 2 Hours
Max. Marks : 40
General Instructions :
- All questions are compulsory.
- The question paper has three sections and 15 questions. All questions are compulsory.
- Section-A has 7 questions of 2 marks each; Section-B has 6 questions of 3 marks each; and Section-C has 2 case based questions of 4 marks each.
- Internal choices have been provided in some questions. A student has to attempt only one of the alternatives in such questions.
Section – A
Question 1.
Answer the following questions: (2)
(a) Define the resistance of a conductor.
(b) State Ohm’s law.
(c) State Joule’s law of heating.
OR
Answer the following questions:
(a) Why are the heating elements of electric toasters and electric irons made of an alloy rather than a pure metal?
(b) Should the resistance of a voltmeter be low or high? Give reason.
Answer:
(a) The obstruction offered to the flow of current by a conductor is called its resistance.
(b) According to Ohm’s law, the current flowing in a conductor is directly proportional to the potential difference applied across its ends, provided the temperature and other physical conditions of the conductor remain constant.
(c) Joule’s law of heating states that the amount of heat produced in a conductor is directly proportional to:
(i) Square of current (I<sup>2</sup>)
(ii) Resistance of wire (R)
(iii) Time (t), for which current is passed.
OR
(a) The resistivity of an alloy is generally higher than that of its constituent metals. Alloys do not oxidise (burn) readily at higher temperatures. Therefore, conductors of electric heating devices, such as toasters and electric irons, are made up of an alloy rather than pure metal.
(b) The resistance of a voltmeter should be high, because voltmeter is connected parallel to the component of a circuit and it also takes negligible current from the circuit in order to measure the potential difference accurately.
Question 2.
Study the diagram and answer the following questions: (2)
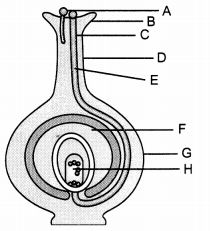
(a) What does the figure indicate?
(b) Label the parts A, B, C, D, E, F, G, H.
(c) Mention the role of parts B, E?
OR
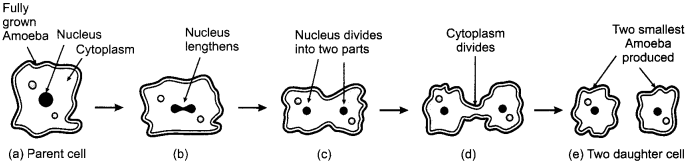
Draw in sequence (showing the four stages), the process of binary fission in amoeba.
Answer:
(a) The figure indicates fertilisation process in flowering plants.
(b) A – Pollen grains, B – Stigma, C – Male gametes, D – Style, E – Pollen tube, F – Ovule, G – Ovary, H – Embryo sac
(c) B – Stigma is the part of carpel which receives pollen grains during pollination.
E – Pollen tube contains the male gamete which passes through the style and finally reaches the ovary. It carries the male gametes towards female gametes for fertilisation.
OR
Binary fission is an asexual mode of reproduction in amoeba where a single parent cell divides into two daughter cells and each daughter cell receives a copy of genetic material.
Question 3.
You have been selected to talk on ‘Ozone layer and its protection’ in the school ‘Assembly on Environment Day’.
(a) Why should ozone layer be protected to save the environment?
(b) List any two ways that you would stress in your talk to bring in awareness among your fellow friends that would also help in protection of ozone layer as well as the environment. (2)
OR
(a) Write the full name of the group of compounds mainly responsible for the depletion of ozone layer?
(b) In a food chain of frog, grass, insect and snake assign trophic level to frog.
Answer:
(a) Ozone layer is present in stratosphere which prevents the ultra-violet rays from sun to penetrate the
Earth’s surface. But due to depletion of ozone layer ultra-violet rays enter into the surface of Earth and cause many health hazards like skin cancer, cataract in eyes etc. So, it is necessary to save the environment by protecting the ozone layer.
(b) Some of the ways to protect the ozone layer are:
1. Banning the use of CFC’s and other ozone depleting substances.
2. Reducing the use of fluorescent lights, limited use of supersonic planes, control over large scale nuclear explosions etc.
OR
(a) CFCs (Chlorofluorocarbons) are mainly responsible for the depletion of ozone layer.
(b) Frog will be at third trophic level.
Grass → Insect Frog → Snake
Question 4.
Answer the following: (2)
(a) Who are more closely related— A brother and sister or two cousins? Why?
(b) Why do all the gametes formed in human females have an X-chromosome?
Answer:
(a) A brother and sister are more closely related than two cousins as they have a common ancestor.
(b) The sex chromosome in human female is homomorphic i.e., they contain same chromosome XX. During meiosis process at the time of gamete formation all egg cell will get one copy of X chromosome, hence all the gametes formed in human females have an X chromosome.
Question 5.
The atomic numbers of three elements A, B and C are 12, 18, and 20 respectively. State giving reason, which two elements will show similar properties. (2)
Answer:
The electronic configuration of A, B and C are as follows:
A → 2, 8,2
B → 2, 8,8
C → 2,8,8,2
Thus, A and C will show similar properties due to same number of valence electrons i.e., 2 in the outermost shell.
Question 6.
Answer the following questions: (2)
(a) Which of the following belongs to homologous series of alkynes?
\(\mathrm{C}_{6} \mathrm{H}_{6}, \mathrm{C}_{2} \mathrm{H}_{6}, \mathrm{C}_{2} \mathrm{H}_{4}, \mathrm{C}_{3} \mathrm{H}_{4}\)
(b) What is a homologous series? Explain with an example.
Answer:
(a) Alkynes have general formula, CnH2n-2, where n is number of carbon atoms. Thus, from the given
options, C3H4 belongs to the homologous series of alkynes.
(b) A homologous series is a series of carbon compounds that have different number of carbon atoms but contain the same functional group. There is a difference of -CH2 unit between each successive member and mass differ by 14u. For example, methane, ethane, propane, butane, etc., are all part of the alkane homologous series. The general formula of this series is CnH2n+2
Question 7.
Identify the process depicted in the diagram given below. What is the advantage of thick wall around spores? (2)
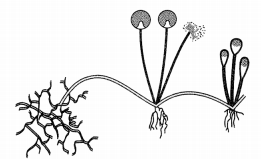
Answer:
The process is known as spore formation in Rhizopus. Spores are covered by a thick wall that protect them until they come into contact with another moist surface and can begin to grow.
Section – B
Question 8.
Answer the following questions: (3)
| Period | Group 1 | Group 2 |
| 1. | A(3) | E(4) |
| 2 | B(11) | F(12) |
| 3. | C(19) | G(20) |
| 4. | D(37) | H(38) |
(a) What is the electronic configuration of F?
(b) What is the number of valence electrons in the atom of F?
(c) Write the size of the atoms of E, F, G and FI in decreasing order.
(d) Out of B, E and F which one has the biggest atomic size?
Answer:
(a) ‘F’ has electronic configuration 2, 8, 2.
(b) ‘F’ has two valence electrons.
(c) H > G > F > Eis decreasing order of size of atoms.
(d)’B’ has the biggest atomic size among B, E and F because atomic size increases from top to bottom in a group.
Question 9.
Answer the following questions: (3)
(a) What do you mean by overloading?
(b) Define an electromagnet.
(c) What is a galvanometer?
Answer:
(a) Overloading is the process of overheating of a wire due to excess current drawn by all the appliances than the permitted limit for that wire. An electromagnet is a magnet consisting of a long coil of insulated copper wire wrapped around a soft iron core that is magnetized only when electric current is passed through the coil.
(b) A galvanometer is an instrument which can detect the presence of electric current in a circuit.
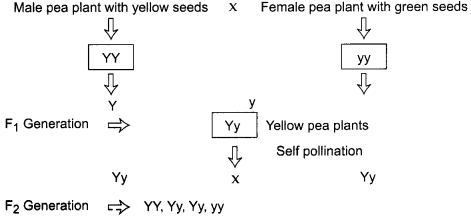
Question 10.
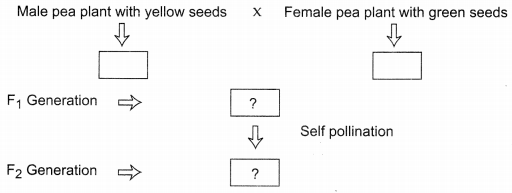
Study carefully the given flowchart depicting cross between pea plant with yellow seeds and pea plant with green seeds and answer the following: (3)
(a) What kind of cross it depicts?
(b) In which proportions the characters will appear in F1 and F2 generation?
Answer:
(a) It depicts monohybrid cross.
(b) In Fa generation all pea plants with yellow seeds will be produced whereas in F2 generation pea plants with yellow and green seeds in the ratio 3 :1 will be produced.
Question 11.
Answer the following questions: (3)
(a) What is a group in the periodic table? In which part of a group would you separately expect the elements to have:
(i) The greatest metallic character.
(ii) The largest atomic size.
(b) In what respects do the properties of group 1 elements differ from those of group 17 elements?
(c) From the stand point of atomic structure, what determines which element will be the first and which is the last in a period of the periodic table?
OR
Explain the periodicity of following properties of elements:
(a) Atomic radius
(b) Metallic character
(c) Electronegativity
Answer:
(a) The vertical columns in a periodic table are called groups.
(i) The greatest metallic character is found in the elements in the lowest part of the group.
(ii) The largest atomic size is found in the lowest part of the group.
(b) Group 1 elements have 1 valence electron and are ionic and electropositive in nature in the chemical reactions, whereas, the elements of group 17 have 7 valence electrons. They all are non-metals and are electronegative in nature.
(c) The number of valence electrons in the atoms of elements decides which element will be the first element in a period and which will be the last in a period.
OR
(a) Atomic radius:
In a period, atomic radius generally decreases from left to right. In a period there is a gradual increase in the nuclear charge. Since valence electrons are added in the same shell, they are more and more strongly attracted towards nucleus. This gradually decreases atomic radii. Atomic radii increases in a group from top to bottom. As we go down a group the number of shells increases and valence electrons are present in higher shell and the distance of valence electrons from nucleus increases. Both the factors decreases the force of attraction between nucleus and valence electron. Therefore, atomic size increases on moving down a group.
(b) Metallic character:
The metallic character of an element can be defined as how readily an atom can lose an electron. Metallic character increases moving down a periodic table group and decreases moving across a period. This is because on moving down a group, atoms add electron shells so the atomic radius increases and it takes less energy to remove electrons. On the other hand, on moving from left to right across a period (not including the noble gases), the number of protons increases but the number of electron shells remains the same. This increases the effective nuclear force on electrons and makes it more difficult to remove them.
(c) Electronegativity:
Electronegativity is relative tendency of a bonded atom to attract the bond- electrons towards itself. Electronegativity decreases in group from top to bottom. In a period, electronegativity increases from left to right because atomic size decreases.
Question 12.
(a) Name the wastes which are generated in your house daily. What measures would you take for their disposal?
(b) What are the advantages of paper bags over plastic bags during shopping?
(c) Pesticides added to the field are seen in increased amounts in the crop and in the birds that feed on them. What is this phenomenon called?
OR
What is an ecosystem? What are the components of an ecosystem? Also discuss the types of ecosystem? (3)
Answer:
(a) The wastes which are generated in our house daily are peels of vegetables and fruits, old and used newspaper, old plastics containers, broken glass, cups, plates, polythene bags, left over foods, Old clothes, toys, utensils, etc.
Measures that should be taken for the disposal of household waste are:
- Biodegradable and non-biodegradable substances should be separated and disposed separately. Kitchen wastes can be used to make compost.
- Safe disposal of plastic and polythene bags.
- Old clothes, toys, utensils etc., can be reused by giving to poor and needy people.
- Plastic, newspapers, polythene and glass apparatus can be recycled by used proper recycling techniques.
(b) The advantages of paper bags are:
- They are made up of biodegradable material.
- They do not cause any environmental pollution.
- They can be recycled and reuse.
(c) Pesticides added to the field are seen in increased amounts in the crop and in the birds that feed on them. This phenomenon is called biological magnification. It is the increasing build-up of toxic substances within organisms that happens at each stage of the food chain. The build-up of toxic substances within a single organism is called biological accumulation.
OR
An ecosystem is the structural and functional unit of biosphere where there is an interaction between living and non-living components to maintain balance between them.
The components of ecosystem are:
1. Biotic components: They are the living organisms like plants, animals, human beings etc. Based on the mode of obtaining food they are classified as producers, consumers and decomposers.
(a) Producers: They are autotrophs which have the capacity to prepare their own food by trapping solar energy and converting them to chemical energy in the form of carbohydrates by the process of photosynthesis.
(b) Consumers: They depend upon.producers for food either directly or indirectly. They can be primary, secondary, tertiary or quaternary consumers.
(c) Decomposers: They obtain food from dead and decayed organisms by breaking them down into simpler forms.
2. Abiotic components:
They are the non-living components i.e., the physical factors like temperature, light, wind, water, humidity and edaphic factors like soil, minerals etc.
The two types of ecosystem are:
1. Natural ecosystem: They are made by nature themselves without human interference. They can be terrestrial like forest, grassland and desert ecosystem and aquatic like freshwater and marine ecosystem.
2. Artificial ecosystem: They are made and maintained by human beings. Examples-Gardens, parks, croplands etc.
Question 13.
An electric kettle is rated at (1000 W – 220 V). (3)
(a) What is the resistance of its element when in use?
(b) What is the safe value of current that can pass through its element?
(c) Two lamps, one rated 100 W at 220 V, and the other 60 W at 220 V, are connected in parallel to electric mains supply. What current is drawn from the line if the supply voltage is 220 V?
Answer:
Here, Power rating, P = 1000 W
Voltage rating, V = 220 V
(a) Using the relation
P = \(\frac{\mathrm{V}^{2}}{\mathrm{R}}\)
Resistance of element when in use,
R = \(\frac{\mathrm{V}^{2}}{\mathrm{P}}\)
= \(\frac{220 \times 220}{1000}\)
= 48.5
(b) Using the relation P = VI
Safe current,
I = \(\frac{P}{V}\)
\(\frac{1000}{220}\)
= 4.55
(c) Given: Power of one lamp, P1 = 100 W Power of second lamp, P2 = 60 W
Since, both the lamps are connected in parallel, thus, potential difference will be equal.
Thus, Potential difference = 220 V
We know, that Power (P) = VI
Thus, the total current through the circuit
I = \(\frac{\mathrm{P}_{1}}{\mathrm{~V}}+\frac{\mathrm{P}_{2}}{\mathrm{~V}}\)
I = \(\frac{100}{220}+\frac{60}{220}\)
= \(\frac{100+60}{220}\)
= \(\frac{160}{220}\)
= 0.727 A
Section – C
Question 14.
(a) Derive the relation \(R=R_{1}+R_{2}+R_{3}\) when resistors are joined in series.
(b) A given length of a wire is doubled on itself. By what factor does the resistance of the wire change?
(c) Derive the relation \(\frac{1}{\mathrm{R}}=\frac{1}{\mathrm{R}_{1}}+\frac{1}{\mathrm{R}_{2}}+\frac{1}{\mathrm{R}_{3}}\) when resistors are joined in parallel.
OR
Out of 60 W and 40 W lamps, which one has a higher electrical resistance when in use. (4)
Answer:
(a) In series combination, the same current flows in all the resistances but the potential difference across each of the resistance is different.
According to Ohm’s law, we have
V1 = IR1, V2 = IR2, V3 = IR3
If the total potential difference between A and B is V, then
V = V1 + V2 + V3
= IR1 + IR2 + IR3
= I(R1 + R2 + R3)
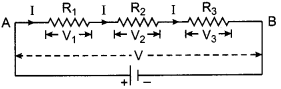
Let the equivalent resistance be R, then
V = IR
and hence
IR = = I(R1 + R2 + R3)
⇒ R = (R1 + R2 + R3)
(b) When given length of wire is doubled on itself, its new length L’ = \(\frac { L }{ 2 }\) and new cross-section area A’ = 2 A. Hence, its new resistance
\(\mathrm{R}^{\prime}=\frac{\rho \mathrm{L}^{\prime}}{\mathrm{A}^{\prime}}=\frac{\rho\left(\frac{\mathrm{L}}{2}\right)}{(2 \mathrm{~A})}=\frac{1}{4} \frac{\rho \mathrm{L}}{\mathrm{A}}=\frac{\mathrm{R}}{4}\)
Thus, resistance is reduced to one-fourth of its original value.
(c) In parallel combination of three resistance R1, R2 and R3, the current in each of the resistance is different. If I is the current drawn from the cell then it is divided into branches as I1, I2 and I3. Thus,
I = I1 + I2 + I3
The potential difference across each of these resistances is the same.
Thus, from Ohm’s law
\(\mathrm{I}_{1}=\frac{\mathrm{V}}{\mathrm{R}_{1}}, \mathrm{I}_{2}=\frac{\mathrm{V}}{\mathrm{R}_{2}}, \mathrm{I}_{3}=\frac{\mathrm{V}}{\mathrm{R}_{3}}\)
If R is the equivalent resistance then,
I = \(\frac { V }{ R }\)
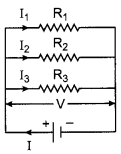
By equation (i), we get
\(\frac{V}{R}=\frac{V}{R_{1}}+\frac{V}{R_{2}}+\frac{V}{R_{3}}\)
and
\(\frac{1}{\mathrm{R}}=\frac{1}{\mathrm{R}_{1}}+\frac{1}{\mathrm{R}_{2}}+\frac{1}{\mathrm{R}_{3}}\)
Power (P) = \(\frac{\mathrm{V}^{2}}{\mathrm{R}}\)
From the above formula, P is inversely proportional to R (resistance) as voltage remaining the same. Hence, 40 W lamp has high resistance.
Question 15.
Our understanding of how inherited traits are passed between generations comes from principles first proposed by Gregor Mendel in 1866. Mendel worked on pea plants, but his principles apply to traits in plants and animals they can explain how we inherit our eye colour, hair colour and even tongue-rolling ability. Mendel found that paired pea traits were either dominant or recessive. When pure-breed parent plants were cross-breed, dominant traits were always seen in the progeny, whereas recessive traits were hidden until the first-generation (F1) hybrid plants were left to self-pollinate.
(a) Will all organisms from a cross between two homozygous dominant organisms would have the same phenotype?
(b) What proportion of offspring from BB v Bb would be BB?
(c) What proportion of offspring from Cc v Cc would be cc?
OR
What proportion of offspring from tt v Tt would be tt? What proportion of offspring from two homozygous recessive organisms would be homozygous recessive? (4)
Answer:
(a) True, all organisms from a cross between two homologous dominant organisms would have the same phenotype which would be homozygous dominant.
(b) 50% of offspring would be BB (homozygous dominant) and the other 50% would be Bb (heterozygous).
(c) One in four (25%) of offspring would be cc (homozygous recessive). Another 25% would be CC (homozygous dominant) and the final 50% would be Cc (heterozygous).
OR
50% of offspring would be tt (homozygous recessive) and the other 50% would be Tt (heterozygous). All offspring (100%) would be homozygous recessive. Without a dominant gene or genes offspring cannot result in heterozygous or homozygous dominant.