Learninsta presents the core concepts of Biology with high-quality research papers and topical review articles.
Mechanism of Muscle Contraction
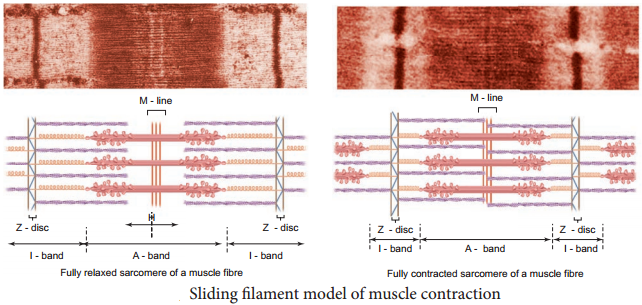
Sliding Filament Theory:
In 1954, Andrew F. Huxley and Rolf Niedergerke proposed the sliding-filament theory to explain muscle contraction. According to this theory, overlapping actin and myosin filaments of fixed length slide past one another in an energy requiring process, resulting in muscle contraction. The contraction of muscle fire is a remarkable process that helps in creating a force to move or to resist a load.
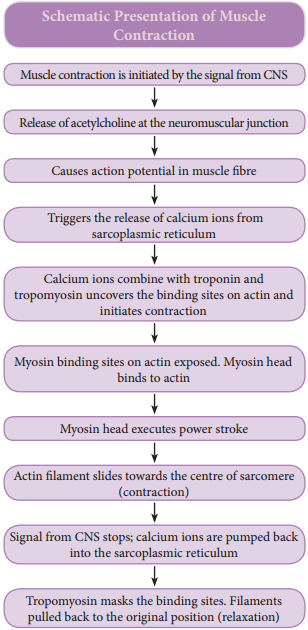
The force which is created by the contracting muscle is called muscle tension. The load is a weight or force that opposes contraction of a muscle. Contraction is the creation of tension in the muscle which is an active process and relaxation is the release of tension created by contraction. Muscle contraction is initiated by a nerve impulse sent by the central nervous system (CNS) through a motor neuron.
The junction between the motor neuron and the sarcolemma of the muscle fire is called the neuromuscular junction or motor end plate. When nerve impulse reaches a neuromuscular junction, acetylcholine is released. It initiates the opening of multiple gated channels in sarcolemma. The action potential travels along the T-tubules and triggers the release of calcium ions from the sarcoplasmic reticulum.
The released calcium ions bind to troponin on thin filaments. The tropomyosin uncovers the myosin-binding sites on thin filaments. Now the active sites are exposed to the heads of myosin to form a cross-bridge (Figure 9.3).
During cross-bridge formation acting and myosin form a protein complex called actomyosin. Utilizing the energy released from hydrolysis of ATP, the myosin head rotates until it forms a 90° angle with the long axis of the filament. In this position myosin binds to an actin and activates a contraction – relaxation cycle which is followed by a power stroke.
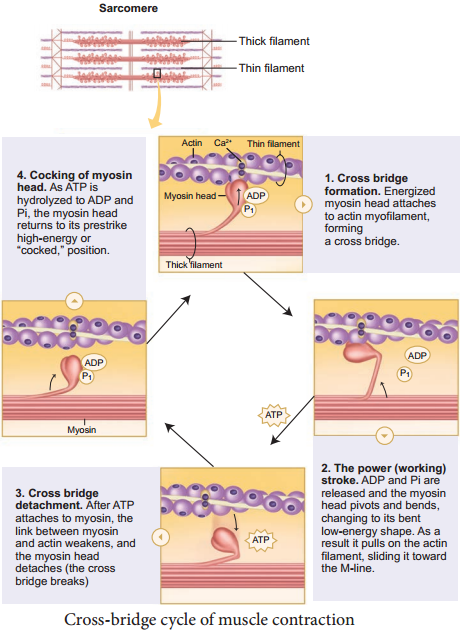
The power stroke (cross-bridge tilting) begins after the myosin head and hinge region tilt from a 90° angle to a 45° angle. The crossbridge transforms into strong, high-force bond which allows the myosin head to swivel. When the myosin head swivels it pulls the attached acting filament towards the centre of the A-band.
The myosin returns back to its relaxed state and releases ADP and phosphate ion. A new ATP molecule then binds to the head of the myosin and the cross-bridge is broken. At the end of each power stroke, each myosin head detaches from actin, then swivels back and binds to a new actin molecule to start another contraction cycle.
This movement is similar to the motion of an oar on a boat. At the end of each power stroke, each myosin head detaches from actin, then swivels back and binds to a new actin molecule to start another contraction cycle. The power stroke repeats many times until a muscle fire contracts.
The myosin heads bind, push and release actin molecules over and over as the thin filaments move toward the centre of the sarcomere. The repeated formation of cross-bridge cycles cause the sliding of the filaments only but there is no change in the lengths of either the thick or thin filaments.
The Z – discs attached to the actin filaments are also pulled inwards from both the sides, causing the shortening of the sarcomere (i.e. contraction). This process continues as long as the muscle receives the stimuli and with a steady flow of calcium ions.
When motor impulse stops, the calcium ions are pumped back into the sarcoplasmic reticulum which result in the masking of the active sites of the actin filaments. The myosin head fails to bind with the active sites of actin and these changes cause the return of Z – discs back to their original position, i.e. relaxation. (Figure 9.4)