Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Introduction of Equilibrium
In our daily life, we observe several chemical and physical changes. For example, a banana gets ripened after few days, silver gets tarnished in few months and iron gets rusted slowly. These processes proceed in one direction.
Now let us consider the transport of oxygen by hemoglobin in our body as an illustration for a reversible change. The hemoglobin combines with oxygen in lungs to form oxyhemoglobin. The oxyhemoglobin has a tendency to form hemoglobin by releasing oxygen. In fact, in our lungs all the three species coexist.
Few chemical reactions proceed in only one direction whereas many reactions proceed in both the directions and these reactions are called reversible reactions. In chemical reactions, the concentration of the reactants decreases and that of the products increases with time. In reversible reactions, initially the reaction proceeds towards the formation of the product.
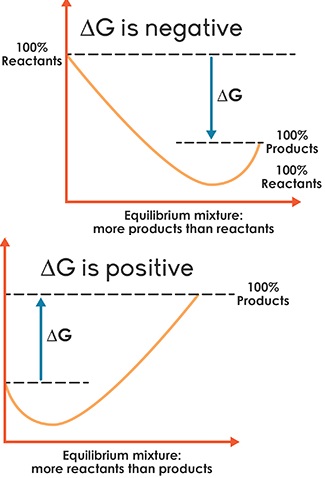
Upon formation of the product, the reverse reaction begins to take place. At a particular stage, the rate of the reverse reaction is equal to that of the forward reaction indicating a state of equilibrium. It is desirable to know the three crucial aspects of chemical reactions namely the feasibility, the rate of the reaction and the extent of reaction.
![]()
We know that the feasibility of a reaction is given by thermodynamics. Chemical kinetics will tell about the rate of the reaction. The equilibrium constant tells about the extent of a reaction which we will discuss in this chapter. We will also discuss the types of equilibrium, the significance of equilibrium constant and its relationship to thermodynamic quantities and the response of chemical equilibrium to change in the reaction conditions.
At equilibrium, with both the forward and reverse reactions taking place at the same rate, the concentration of every species no longer changes. Every reaction has a point in which equilibrium is established. But for other reactions, equilibrium occurs when only part of the reactants are converted into products.
Equilibrium is the state in which market supply and demand balance each other, and as a result prices become stable. Generally, an over-supply of goods or services causes prices to go down, which results in higher demand-while an under-supply or shortage causes prices to go up resulting in less demand.
A state of rest or balance due to the equal action of opposing forces. equal balance between any powers, influences, etc.; equality of effect. mental or emotional balance; equanimity: The pressures of the situation caused her to lose her equilibrium.
Equilibrium always happens at the same point in the reaction no matter where you start. They reach equilibrium at the same point whether you start with all A, all B/C, or half A and half B/C. It doesn’t matter. There is one special point where the forward and reverse reactions cancel each other out.
Equilibrium is important to create both a balanced market and an efficient market. If a market is at its equilibrium price and quantity, then it has no reason to move away from that point, because it’s balancing the quantity supplied and the quantity demanded.
Another way of defining equilibrium is to say that a system is in equilibrium when the forward and reverse reactions occur at equal rates. Equilibrium does not necessarily mean that reactants and products are present in equal amounts.
![]()
An example of equilibrium is when you are calm and steady. An example of equilibrium is when hot air and cold air are entering the room at the same time so that the overall temperature of the room does not change at all.
A fish is an ideal example of equilibrium. A fish swimming at a certain depth under the sea without floating upward or sinking unexpectedly is said to be in equilibrium. This is achieved by the swim bladder present in it. The bladder is present in the belly of a fish.
Explanation:
A mixture is a substance in which two or more components are mixed together. For example, sugar in water, mud in water etc are all mixture. Whereas an equilibrium mixture is a mixture in which components of both reactants and products are present in ratio with each other.
Equilibrium is important to create both a balanced market and an efficient market. If a market is at its equilibrium price and quantity, then it has no reason to move away from that point, because it’s balancing the quantity supplied and the quantity demanded.