Students can access the CBSE Sample Papers for Class 12 Chemistry with Solutions and marking scheme Set 6 will help students in understanding the difficulty level of the exam.
CBSE Sample Papers for Class 12 Chemistry Set 6 with Solutions
Time Allowed: 3 hours
Maximum Marks: 70
General Instructions:
- There are 33 questions in this question paper with internal choice
- SECTION A consists of 16 multiple-choice questions carrying 1 mark each.
- SECTION A consists of 16 multiple-choice questions carrying 1 mark each.
- SECTION C consists of 7 short answer questions carrying 3 marks each.
- SECTION D consists of 2 case-based questions carrying 4 marks each.
- SECTION E consists of 3 long answer questions carrying 5 marks each.
- All questions are compulsory.
- Use of log tables and calculators is not allowed.
SECTION – A (16 Marks)
The following questions are multiple-choice questions with one correct answer. Each question carries 1 mark. There is no internal choice in this section.
Question 1.
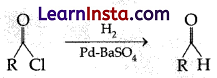
The catalyst used in Rosenmund’s reduction is:
(a) HgSO4
(b) Pd/BaSO4
(c) anhydrous AlCl3
(d) anhydrous ZnCl2
Answer:
(b) Pd/BaSO4
Explanation: A catalyst used in Rosenmund’s reaction can be prepared by reduction of palladium(II) chloride solution in the presence of BaSO4.
The palladium catalyst used in Rosenmund’s reduction must be poisoned with BaSO4 because the untreated catalyst Palladium (Pd) is too reactive and will undergo over reduction forming other products
Question 2.
Match the components/elements given in Column I with uses given in Column II:
| Column I | Column II | ||
| (a) | Lanthanoid oxide | (1) | Production of iron alloy |
| (b) | Lanthanoid | (2) | Television screen |
| (c) | Misch metal | (3) | Petroleum cracking |
| (d) | Magnesium based alloy | (4) | Lanthanoid metal + iron |
| (e) | Mixed oxides of lanthanoids are employed | (5) | Lanthanoid metal + iron |
(a) (a)-(2), (b)-(l), (c)-(4), (d)-(5), (E)-(3)
(b) (a)-(3), (b)-(4), (c)-(5), (d)-(l), (e)-(2)
(c) (a)-(5), (b)-(l), (c)-(5), (d)-(3), (E)-(4)
(d) (a)-(l), (b)-(5), (c)-(2), (d)-(4), (e)-(3)
Answer:
(a) (a)-(2), (b)-(l), (c)-(4), (d)-(5), (E)-(3)
Explanation: Lanthanoid oxide is used in TV screens, Lanthanoid are used in production of iron alloy, Misch metal consists of lanthanoid metal + iron, Mischmetal is used in Mg-based alloy to produce bullets, shell and lighter flint and mixed oxides of lanthanoids are employed in petroleum cracking.
Question 3.
Structure of a disaccharide formed by glucose and fructose is given below. Identify anomeric carbon atoms in monosaccharide units.
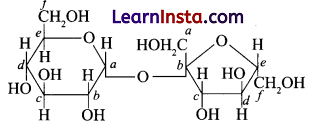
(a) ‘a’ carbon of glucose and ‘a’ carbon of fructose.
(b) ‘a’ carbon of glucose and ‘e’ carbon of fructose.
(c) ‘a’ carbon of glucose and ‘b’ carbon of fructose.
(d) ‘f’ carbon of glucose and ‘f’ carbon of fructose.
Answer:
(c) ‘a’ carbon of glucose and ‘b’ carbon of fructose.
Explanation: In a cyclic structure of glucose or fructose, C atom which is adjacent to oxygen atom is known as anomeric carbon. In the given structures a and b on C which are present adjacent to O atom are anomeric. Both anomeric carbons differ in the configuration of -OH group.
![]()
Question 4.
Reduction of CH3CH2NC with hydrogen in presence of Ni or Pt as catalyst gives:
(a) CH3CH2NH2
(b) CH3CH2NHCH3
(c) CH3CH2NHCH2CH3
(d) (CH3)3N
Answer:
(b) CH3CH2NHCH3
Explanation: The reduction of isonitrile with hydrogen in presence of Ni or Pt is known as catalytic hydrogenation. The — NC is known as isonitrile. In isonitrile, the negative charged carbon work as a nucleophile. It attacks hydrogen and gets protonated. The triple bond shifts to the nitrogen and the lone pair of the nitrogen attacks on another hydrogen and the breaking of the N = C bond results in the secondary amine. Thus, the reduction of CH3CH2NC with hydrogen in presence of Ni or Pt as catalyst gives CH3CH2NHCH3.
Question 5.
What is the IUPAC name of

(a) 2-dimethylchloropropane
(b) 1-chloreo-2-dimethyl-pentane
(c) 2, 2-dimethyl-chlorobutane
(d) 1-chloro-2, 2-dimethyl propane
Answer:
(d) 1-chloro-2, 2-dimethyl propane
Explanation: According to IUPAC Nomenclature, the name of the given compound is 1-chloro-2, 2- dimethyl propane.
Question 6.
A fruity smell is produced by the reaction of C2H5OH with:
(a) PCl5
(b) CH3COCH3
(c) CH3COOH
(d) None of these
Answer:
(c) CH3COOH
Explanation: Ester can be identified by fruity smell at the end of reaction. When alcohol is treated with add, alcohol gets protonated via H2SO4 and attacks acid thus, formation of Ester and removal of water molecule can take place. Ester can be identified by fruity smell at the end of reaction.

Question 7.
The standard electrode potentials of four elements A, B, C and D are -3.05, 1.66, -0.40 and 0.80 volts respectively. The highest chemical activity will be shown by:
(a) A
(b) B
(c) C
(d) D
Answer:
(a) A
Explanation: Lower the reduction potential better will be reactivity or chemical activity. Among A, B, C and D, Standard electrode potential i.e,, reduction potential of A is minimum (-3.05V) i.e., its oxidation potential is maximum which implies ‘A’ has maximum chemical reactivity.
Question 8.
Identify the correct naming for K3[Fe(CN)6].
(a) Tripotassium hexacyanidoferrate(III)
(b) Potassium hexacyanoferrate(II)
(c) Tripotassium hexacyanoferrate(III)
(d) Potassium hexacyanidoferrate(III)
Answer:
(d) Potassium hexacyanidoferrate(III)
Explanation: According to the IUPAC nomenclature, the IUPAC name of K3[Fe(CN)6] is potassium hexacyanidoferrate (III).
Question 9.
Consider a reaction aG + bH → products. When concentration of both the reactants G and H is doubled, the rate increases by eight times. However, when concentration of G is doubled keeping the concentration of H fixed, the rate is doubled. The overall order of the reaction is:
(a) 0
(b) 1
(c) 2
(d) 3
Answer:
(d) 3
Explanation: aG + bH → products
Suppose order of reaction = n
When concentration of both C and H doubted then rate increase by eight times.
Rate = K(reactants)n
(8) = K(2)n
(2)3 = K(2)n
n = 3
When concentration of G is doubted keeping the concentration of fixed, the rate is double.
Then, rate ∝ [G]1
rate ∝ [G]1 [H]2.
![]()
Question 10.
The IUPAC name of CH3COCH(CH3)2 is:
(a) 2-methyl-3-butanone
(b) 4-methylisopropyl ketone
(c) 3-methyl-2-butanone
(d) Isopropylmethyl ketone
Answer:
(c) 3-methyl-2-butanone
Explanation: The longest chain is of four carbon. The functional group here is keto group. The numbering will start from left carbon as the functional group should also have the lowest numbering. The substituent methyl group will be at third carbon.
Hence, the IUPAC name of this structure is 3-methyl-2-butanone.
Question 11.
Which of the following has the maximum number of unpaired electrons?
(a) Mg2+
(b) Ti3+
(c) V3+
(d) Fe2+
Answer:
(d) Fe2+
Explanation: Let us compare the electronic configuration of these elements:
Mg2+: 1s22s22p6: no impaired electron.
Ti3+: 1s22s22p63s23p63d1: one impaired electron.
V3+: 1s22s22p63s23p63d2: two unpaired electrons.
Fe2+: 1s22s22p63s2sp63d6: four unpaired electrons.
Thus, we see that Fe2+ has the most unpaired electrons.
Question 12.
When acetaldehyde is heated with Fehling’s solution it gives a precipitate of:
(a) Cu
(b) CuO
(c) Cu2O
(d) CU(OH)2
Answer:
(c) Cu2O
Explanation: Fehlings solution is a tartaric acid complex of cupric ions.
When acetaldehyde is heated with Fehling’s solution, it gives a red precipitate of Cu2O.
\(\mathrm{CH}_3 \mathrm{CHO}+2 \mathrm{Cu}^{2+}+5 \mathrm{OH}^{-} \stackrel{\Delta}{\longrightarrow} \mathrm{CH}_3 \mathrm{COO}^{-}+\mathrm{Cu}_2 \mathrm{O} \downarrow+3 \mathrm{H}_2 \mathrm{O}\)
Acetaldehyde is oxidised to acetate ion and cupric ions are reduced to cuprous oxide.
Question No.13 to 16 consist of two statements – Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
Question 13.
Assertion (A): Ethers behave as bases in the presence of mineral acid.
Reason (R): Due to the presence of lone pair of electrons on the oxygen atom.
Answer:
(a) Both A and R are true and R is the correct explanation of A.
Explanation: Due to the presence of lone pair of electrons on oxygen atom. Ethers behave as base and form stable oxonium salts with mineral acid. Thus, both assertion and reason are correct statements and reason is the correct explanation of assertion.

Question14.
Assertion (A): In presence of enzyme, substrate molecule can be attacked by the reagent effectively.
Reason (R): Active sites of enzymes hold the substrate molecule in a suitable position.
Answer:
(a) Both A and R are true and R is the correct explanation of A.
Explanation: In the presence of enzyme, substrate molecule can be attacked by a reagent effectively because active sites of enzymes hold the substrate molecule in a suitable position. So, enzyme catalysed reactions are stereospecific reactions. Thus, both assertion and reason are correct statements and reason is the correct explanation of assertion.
Question 15.
Assertion (A): Kohlrausch law does not help to find the molar conductivity of weak electrolyte at infinite dilution.
Reason (R): Molar conductivity of a weak electrolyte at infinite dilution cannot be determined experimentally.
Answer:
(d) A is false, but R is true.
Explanation: According to Kohlrausch’s law the molar conductivity of an electrolyte at infinite dilution is equal to the sum of the conductances of the anions and cations.
μ∞ = mλ+∞ + nλ–∞
r = molar conductance at infinite dilution.
λ+, λ– = conductance of cations and anions.
m,n = number of ions formed.
So, Kohlrausch law helps to find molar cooductivity of weak electrolyte and infinite dilutions. The conductance of weak electrolytes is low and even at infinite dilution the dissociation of electolyte is not complete, so the molar conductivity of a weak electrolyte at infinite dilution cannot be determined experimentally. Thus, assertion is wrong statement but reason is correct statement.
![]()
Question 16.
Assertion (A): Tetrahedral complexes do not show geometrical isomerism.
Reason (R): Unidentate ligands attached to the central metal ion are same.
Answer:
(a) Both A and R are true and R is the correct explanation of A.
Explanation: Tetrahedral complexes do not show geometrical isomerism because relative positions of the unidentate ligands attached to the central metal atom are same with respect to each other. This type of isomerism arises in heteroleptic complexes. Thus, both assertion and reason are correct statements and reason is the correct explanation of assertion.
SECTION – B (10 Marks)
This section contains 5 questions with internal choice in one questions. The following questions are very short answer type and carry 2 marks each.
Question 17.
Use Hund’s rule to derive the electronic configuration of Ce3+ ion and calculate its magnetic moment on the basis of ‘spin-only’ formula.
Answer:
Electronic configuration of Ce : Is2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f1 5d1 6s2.
Ce3+ : [Xe] 4f1
Magnetic moment can be calculated as:
μ = \(\sqrt{n(n+2)}\)
Where,
n = Number of unpaired electrons
For
Ce3+ : 54[Xe] 4f1 (only one unpaired electron)
i.e., n = 1
According to ‘spin-only’ formula,
Magnetic moment of Ce3+ (μ) = \(\sqrt{n(n+2)}\) BM
= \(\sqrt{1(1+2)}\) BM = √3 BM = 1.73 BM
Question 18.
(a) Write the major product of the following reaction:
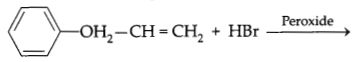
(b) What is Saytzeff rule? Illustrate with suitable example.
OR
(a) What happens when benzyl chloride is boiled with aqueous sodium hydroxide solution?
(b) What is meant by chirality of a compound? Give an example.
Answer:

(b) There are haloalkanes that can undergo elimination in two different ways resulting in two different products. Alkenes with less number of hydrogens on the double-bonded carbon atoms are the preferred product. This process is known as Saytzeff’s rule. According to Saytzeff’s rule in dehydrohalogenation reactions, the preferred product is that alkene which has the greater number of alkyl groups attached to the doubly bonded carbon atoms.
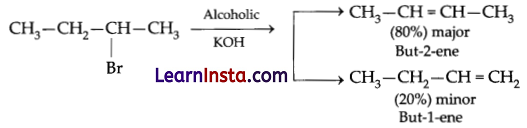
OR
(a) Benzalchloride, undergoes hydrolysis and forms benzaldehyde.
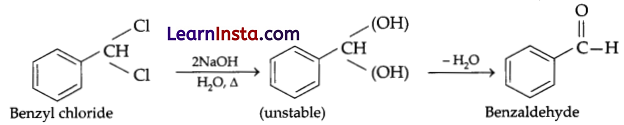
(b) A compound with no point of symmetry and which is non-superimposable on its mirror image is called a chiral compound and the property is called chirality

Question 19.
How will you convert nitrobenzene?
Answer:
Aniline is obtained from nitrobenzene by reduction with Sn/Conc. HCl. The chemical reaction can be represented as follows:
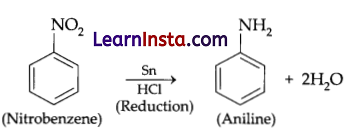
Question 20.
How is equilibrium constant K related to only E°cell and not Ecell ?
Answer:
According to Nernst equation,
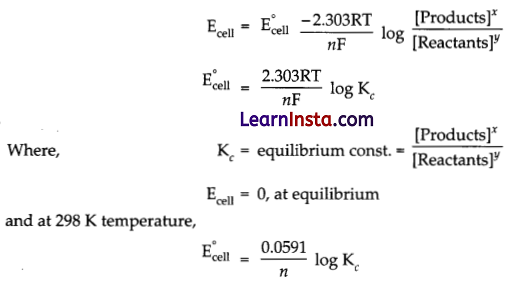
![]()
Hence, equilibrium constant (Kc) is related to only E°cell and not Ecell.
Question 21.
(a) Write the pyranose structure of glucose.
(b) What happens when D-glucose is treated with HNO3?
Answer:
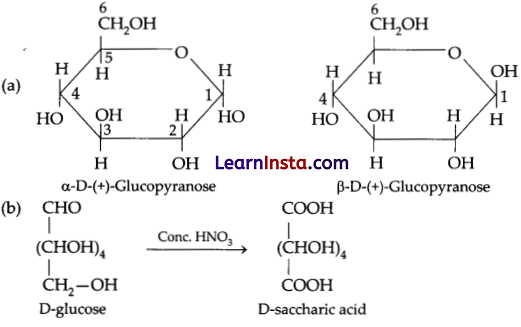
SECTION – C (21 Marks)
This section contains 7 questions with internal choice in one questions. The following questions are short answer type and carry 3 marks each.
Question 22.
Convert the following:
(a) Methyl chloride into ethyl chloride.
(b) Ethyl chloride to propanoic acid.
(c) Diphenyl from chlorobenzene.
Answer:
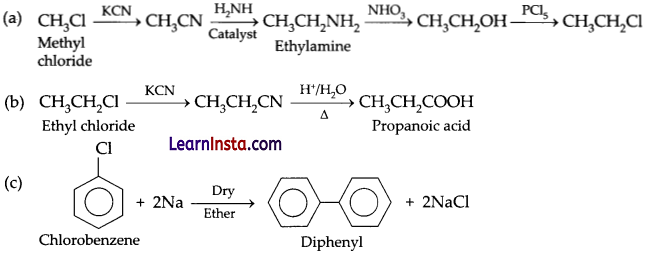
Question 23.
Explain briefly the following reactions. (Any two)
(a) Hofmann’s bromamide reaction
(b) Carbylamine reaction
(c) A coupling reaction
Answer:
(a) Hofmann’s bromamide reaction: When amide is treated with bromide in alkaline solution, and amide yields an amine containing one carbon less than the starting amide.
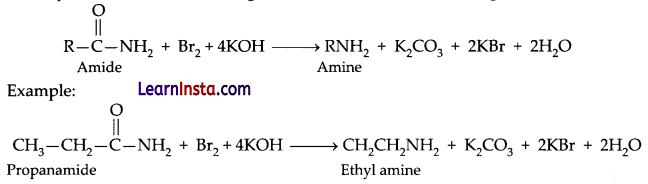
(b) Carbylamine reaction: Also called as isocyanide reaction which is used as a test for the identification of primary amines. When aliphatic and aromatic primary amines are heated with chloroform and ethanolic potassium hydroxide, carbylamines (or isocyanides) are formed. These carbylamines have very unpleasant odours. Secondary and tertiary amines do not respond to this test.
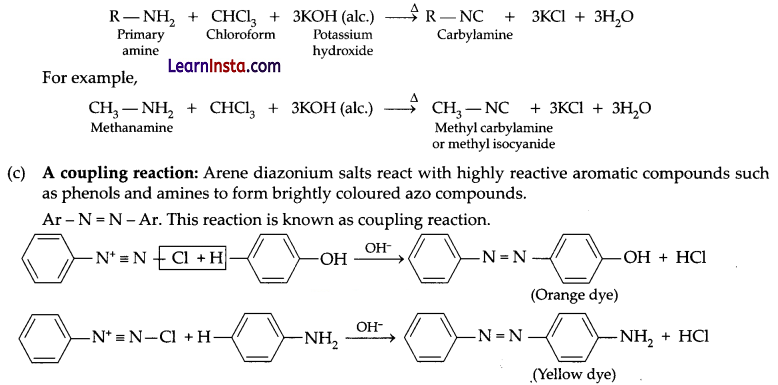
Question 24.
(a) What do you understand by the term glycosidic linkage?
(b) Classify the following into monosaccharides and disaccharides. Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose.
(c) Write one difference between a-helix and β-pleated structures of proteins.
Answer:
(a) Glycosidic linkage refers to the linkage formed between two monosaccharide units through an oxygen atom by the loss of a water molecule.
For example, in a sucrose molecule two monosaccharide units, a-glucose and p-fructose, are joined together by a glycosidic linkage.
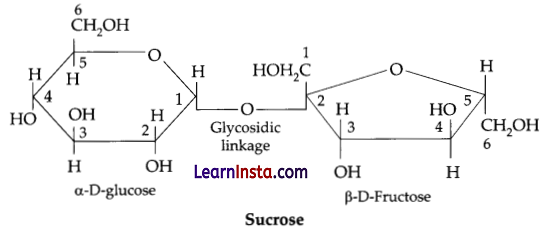
(b) Monosaccharides: Ribose, 2-deoxyribose, Sucrose Galactose, Fructose.
Disaccharides: Maltose, Lactose.
(c) In a-helix structure of proteins, the peptide chains are coiled to form right handed helix involving hydrogen bonding. In p-pleated structure of proteins, the peptide chains lie side by side joined together by inter-molecular hydrogen bonding.
Question 25.
Answer the following questions:
(a) Transition metals generally form coloured compounds.
(b) Why are Mn2+ compounds more stable than Fe2+ towards oxidation to their +3 state?
(c) Silver is a transition metal but zinc is not.
Answer:
(a) Most of the transition metal ions are coloured both in the solid-state and in aqueous solutions. The colour of these ions is attributed to the presence of an incomplete (n- 1) d-subshell. The electrons in these metal ions can be easily promoted from one energy level to another in the same d-subshell. The amount of energy required to excite the electrons to higher energy states within the same d-subshell corresponds to the energy of certain colours of visible light. Therefore, when white light falls on a transition metal compound, some of its energy corresponding to a certain colour is absorbed causing the promotion of d-electrons. This is known as d-d transitions. The remaining colours of white light are transmitted and the compound appears coloured.
(b) The electronic configuration of Mn2+ is [Ar] 3d5 which is half-filled and hence it is stable. Therefore, the third ionization enthalpy is very high, i.e., the third electron cannot be easily removed. In the case of Fe2+, the electronic configuration is 3d6. Therefore, Fe2+ can easily lose one electron to acquire a 3d5 stable electronic configuration.
(c) According to the definition, transition elements are those which have partially filled d-subshell in their elementary state or in one of the oxidation states. Silver (Z = 47) can exhibit a + 2 oxidation state in which it has an incompletely filled d-subshell (4d9 configuration). Hence, silver is regarded as a transition element.
On the other hand, zinc (Z = 30) has the configuration 3d10 4s2. It does not have partially filled d-subshells in its elementary form or in a commonly occurring oxidation state (Zn2+: 3d10). Therefore, it is not regarded as a transition element.
![]()
Question 26.
The following data were obtained during the first order thermal decomposition of SO2Cl2 at a constant volume.
SO2Cl2(g) → SO2 + Cl2(g)
| Experiment | Time(s-1) | Total Pressure(atm) |
| 1 | 0 | 0.5 |
| 2 | 100 | 0.6 |
Calculate the rate of the reaction, when total pressure is 0.65 atm.
Answer:
The thermal decomposition of SO2Cl2 at a constant volume is represented by the following equation :
SO2Cl2(g) → + SO2(g) + Cl2(g)
At t = 0 P0 0 0
At t = t P0 – p p p
After time t, total pressure, Pt = (P0– P) + P + P
Pt = P0 + P
p = pt – p0
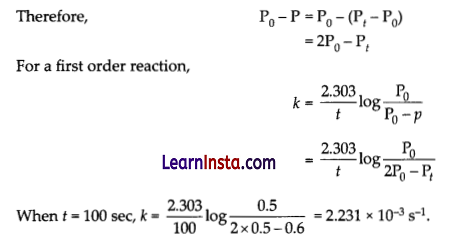
When Pt = 0.65 atm,
P0 + p = 0.65
⇒ p = 0.65 – P0 = 0.65 – 0.5 = 0.15 atm
Therefore, when the total pressure is 0.65 atm, pressure of SO2Cl2 is
SO2Cl2 = P0 – p = 0.5 – 0.15 = 0.35 atm
Therefore, the rate of equation, when total pressure is 0.65 atm, is given by
Rate = k(PSO2Cl2) = (2.23 × 10-3 s-1) (0.35 atm)
= 7.8 × 10-4 atm s-1.
Question 27.
In general, the rate of the chemical reaction doubles for an increase of 10 K in absolute temperature from 298 K. Calculate Ea.
Answer:
It is given that T1 = 298 K
∴ T2 = (298 + 10) K = 308 K
We also know that the rate of the reaction doubled when temperature is increased by 10°.
Therefore, let us take the value of k1 = k and that of k2 = 2k
Also, R = 8.314 JK-1 mol-1.
Now, substituting these values in the equation :
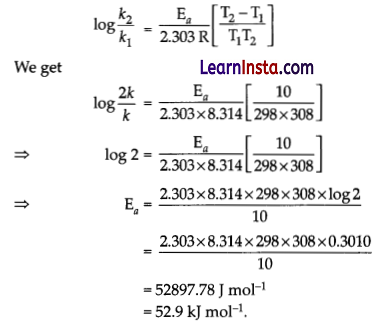
Question 28.
Based on solute-solvent interactions, arrange the following in order of increasing solubility in n-octane and explain. Cyclohexane, KCl, CH3OH, CH3CN.
Answer:
n-octane is a non-polar solvent. Therefore, the solubility of a non-polar solute is more than that of a polar solute in the n-octane.
The order of increasing polarity is:
Cyclohexane < CH3CN < CH3OH < KCl
Therefore, the order of increasing solubility is: KCl < CH3OH < CH3CN < Cyclohexane
SECTION – D (8 Marks)
The following questions are case-based questions. Each question has an internal choice and carries 4 (1+1+2) marks each. Read the passage carefully and answer the questions that follow.
Question 29.
Methyl tert-butyl ether appears as a colourless liquid with a distinctive anesthetic-like odour. Vapours are heavier than air and narcotic. Boiling point 131°F. Less dense than water and miscible in water. Used as an octane booster in gasoline. Methyl tert-butyl ether (MTBE) is a flammable liquid with a distinctive, disagreeable odour. It is made from blending chemicals such as isobutylene and methanol, and has been used since the 1980s as an additive for unleaded gasoline to achieve more efficient burning. Methyl tertbutyl ether is an ether having methyl and tert-butyl as the two alkyl components. It has a role as a non-polar solvent, a fuel additive and a metabolite. After careful investigation it has been found that f-butyl ethyl ether can not be prepared by the following reaction.
C2H5 – ONa + (CH3)3C → Cl (CH3)3C – OC2H5
Answer the following questions:
(a) Why the above-mentioned reaction does not occur?
(b) What would be the major product of the above reaction instead of t-butyl ethyl ether?
(c) Write the mechanism for the formation of major product in the above reaction.
OR
Write a suitable reaction for the preparation of t-butyl ethyl ether.
Answer:
(a) (CH3)3CCl is a tert-alkyl halide. It does not undergo nucleophilic substitution with C2H5O–Na+ due to steric reason.
(b) Isobutene.
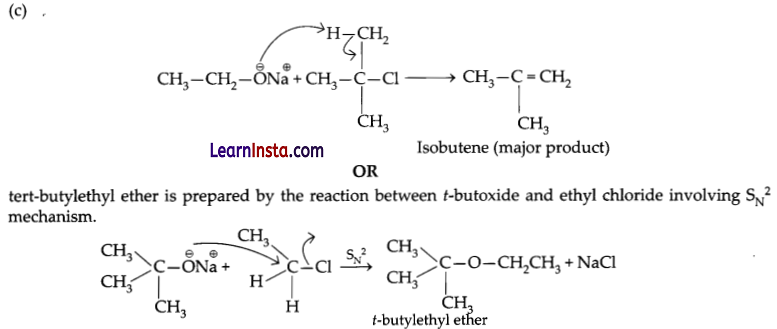
![]()
Question 30.
Van’t Hoff found that electrolytes do not obey osmotic pressure equation (π = cRT). In such cases, π > CRT. Similar situation is observed for all colligative properties. For electrolytes Van’t Hoff modified his equation π = iCRT, ‘i’ is called Vant Hoff factor.
For three different solutions ‘t’ values are tabulated below:
| 0.1M Solution | ‘i’ values |
| A | 1 |
| B | 0.82 |
| C | 1.53 |
Answer the following questions:
(a) Under what condition ‘i’ value is equal to unity?
(b) What would be the value of ‘i’ for a dilute solution of K2SO4 is water?
(c) How ‘i’ value is related to degree of dissociation – Explain.
OR
Under which condition ‘i’ value is >1 and <1 – Explain.
Answer:
(a) When the solute does not undergo any dissociation or association in the solution i.e., for a non-electrolyte solution the value of Y is equal to unity.
(b) The value of i for K2SO4 will be:
K2SO4 → 2k+ + SO42-
i = 3.
(c) For an electrolyte if one molecule dissociates into ‘n’ particles and ‘a’ be the degree of dissociation then after dissociation particles formed from one particle are (1 – α + nα)
This is ‘i’. So, i = 1 – α + n α
OR
When the solute undergo association in the solution ‘i’ becomes < 1 but when the solute undergoes dissociation in the solution ‘i’ becomes > 1.
SECTION – E (15 Marks)
The following questions are long answer type and carry 5 marks each. All questions have an internal choice.
Question 31.
(a) Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their variation with concentration.
(b) The standard electrode potential (E°) for the cell containing 0.1 M Ag+ and 4.00 M Cu2+ at 298 K are
E° Cu2++/Cu = + 0.34V,E° Ag+/Ag = +0.80 V.
Calculate the cell potential (E).
(c) How many hours does it take to reduce 3 mol of Fe3+ to Fe2+ with 2.00 A current? [R = 8.314 J K-1 mol-1 F = 96500 C]
OR
(a) Calculate the standard cell potentials of galvanic cells in which the following reactions take place:
(i) 2Cr(s) + 3Cd2+(aq) → 2Cr3+(aq) + 3Cd E°Cr3+/cr = – 0.74 V
(ii) Fe2+(aq) + Ag+(aq) → Fe3+(aq) + Ag(s) E°Cd2+/Cd = -0,40 V
Calculate the ArG° and equilibrium constant of the reactions.
(b) The molar conductivity of 0.025 mol L-1 methanoic acid is 46.1 S cm2 mol-1.
Calculate its degree of dissociation and dissociation constant. Given λ°(H+) = 349.6 S cm2 mol-1 and λ°(HCOO–) = 54.6 S cm2 mol-1.
Answer:
(a) Conductivity of a solution is defined as the conductance of a solution of 1 cm in length and area of crosssection 1 sq. cm. The inverse of resistivity is called conductivity or specific conductance. It is represented by the symbol K. If Q is resistivity, then we can write :
k = \(\frac{1}{\rho}\) units are Ω-1 cm-1
Conductivity always decreases with a decrease in concentration, both for weak and strong electrolytes. This is because the number of ions per unit volume that carry the current in a solution decreases with a decrease in concentration.
Molar conductivity of a solution at a given concentration is the conductance of volume V of a solution containing 1 mole of the electrolyte kept between two electrodes with the area of cross-section A and distance of unit length.

OR
Answer:
(a) (i) E°cr3+/cr =- 0.74 (Given)
E°Cd2+/Cd = -0.40V
The galvanic cell of the given reaction is depicted as :
Cr(s) | Cr3+(aq) || Cd2+(aq) | Cd(s)
Now, the standard cell potential is
E°cell = E°R – E°L
= – 0.40 – -0.74)
= + 0.34 V
ΔrG° = -nFE°cell
In the given equation, n = 6
F = 96487 C mol-1
E°cell = + 0.34 V
ΔrG° = -6 × 96487 C mol-1 × 0.34 V
= -196833.48 J mol-1
= -196.83 kJ mol-1
Then,
If value of F = 96500 C used then ΔrG° = -196.86 kJ/mol (Both are correct)
Again, ΔrG° =- RT In K
⇒ ΔrG° = -2.303 RT log K
⇒ log K \( \begin{aligned}
& =-\frac{\Delta_r \mathrm{G}}{2.303 \mathrm{RT}} \\
& =\frac{196.83 \times 10^3}{2.303 \times 8.314 \times 298}
\end{aligned}\)
= 34.496
K = antilog (34.496)
= 3.13 × 1034
(ii) E°Fe3+/Fe2+ = 0.77 V
E°Ag+/Ag = 0.77 V
The galvanic cell of the given reaction is depicted as: Fe2+(aq) | Fe3+(aq) || Ag+(aq) | Ag(s)
Now, the standard cell potential is
E°cell = E°R – E°L
= 0.80 – 0.77 = 0.03 V
Here, n = 1.
Then, ΔrG° = -nFE°cell
= -1 × 96487 C mol-1 × 0.03 V
= -2894.61 J mol-1
= -2.89 kJ mol-1
Again, ΔrG° =- 2.303 RT log K
\(\begin{aligned}
\log \mathrm{K} & =\frac{-\Delta_r \mathrm{G}}{2.303 \mathrm{RT}} \\
& =\frac{2894.61}{2.303 \times 8.314 \times 298}
\end{aligned}\)
= 0.5073
K = antilog (0.5073)
= 3.2 (approximately)
(b) C = 0.025 mol L-1
∧m = 46.1 S cm2 mol-1
λ°(H+) = 349.6 S. cm2 mol-1
λ°(HCOO–) = 54.6 S cm2 mol-1
∧°m (HCOOH) = λ°(H+) + λ°(HCOO–)
= 349.6 + 54.6 = 404.2 S cm2 mol-1
Now, degree of dissociation: \(\alpha=\frac{\Lambda_{m(\mathrm{HCOOH})}}{\Lambda_{m(\mathrm{HCOOH})}^{\mathrm{o}}}=\frac{46.1}{404.2}\)
= 0.114 (approximately)
Thus, dissociation constant: k = \(\begin{aligned}
& \frac{c \alpha^2}{(1-\alpha)} \\
& =\frac{0.025 \mathrm{~mol} \mathrm{~L}^{-1}(0.114)^2}{(1-0.114)}
\end{aligned}\)
= 3.67 × 10-4 mol L-1.
![]()
Question 32.
(a) Draw the structures of geometrical isomers of [Fe(NH3)2 (CN)4]–
(b) [NiCl4]2- is paramagnetic while [Ni(CO)4] is diamagnetic though both are tetrahedral. Why? [Atomic number of Ni = 28]
(c) What is a spectrochemical series? How is it formed?
OR
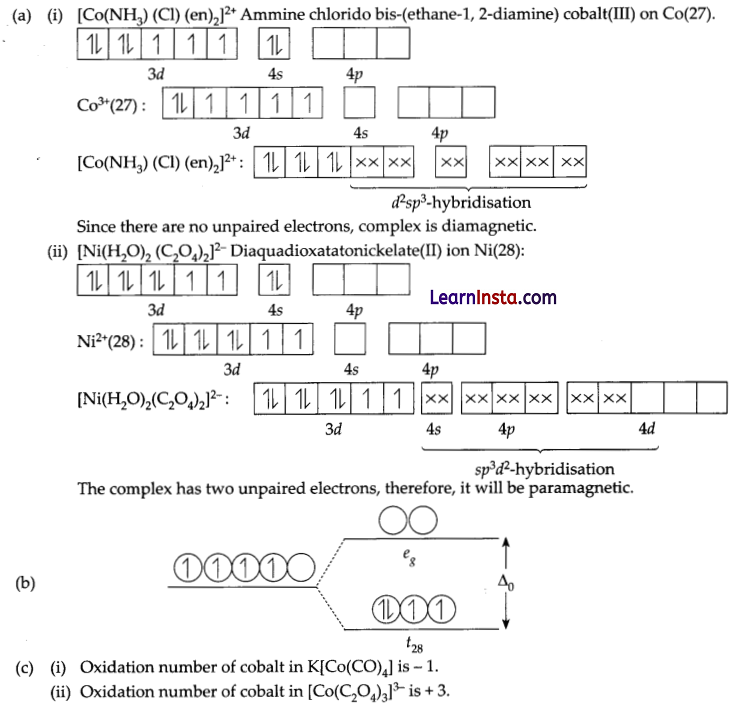
(a) Give the formula of each of the following coordination entities:
(i) CO3+ ion is bound to one Cl–, one NH3 molecule and two bidentate ethylene diamine (en) molecules.
(ii) Ni2+ ion is bound to two water molecules and two oxalate ions. Write the name and magnetic behaviour of each of the above coordination entities. (At. nos. CO = 27, Ni = 28)
(b) On the basis of crystal field theory, write the electronic configuration for d4 ion if Δ0 > P
(c) Predict the oxidation number of cobalt in the following complexes.
(i) K[CO(CO)4]
(ii) [Co(C2O4)3]3-
Answer:
(a) Geometrical isomers of complex [Co(en)2Cl2]+:
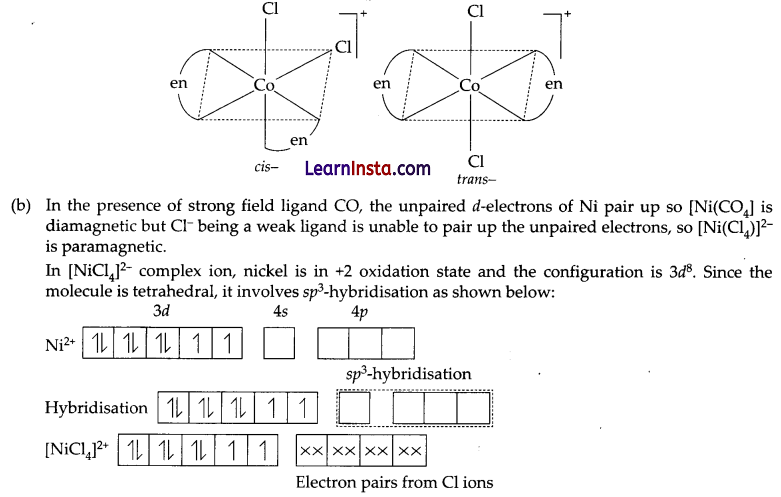
The molecule is paramagnetic because it contains two unpaired electrons.
In [NiCl4] nickel is in O oxidation state and has the configuration 4s2 3d4 or 3d10. The molecule is tetrahedral and involves sp3-hybridisation as given below:
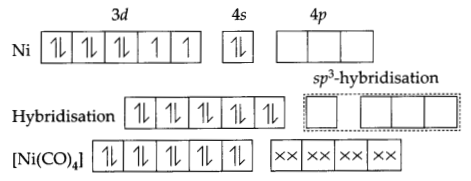
Each CO donates a pair of electrons forming four Ni-CO bonds. The compound is diamagnetic since it contains no unpaired electron.
(c) Spectrochemical series is a series in which the ligands have been arranged in order of increasing field strength.
I– < Br– < SCN < Cl– < S-2 < N3– < F- < ONO– < OH– < SO4-2 < NO4– < C2O4-2 < O-2 < H2O ~ NCS– < EDTA-4 < NH3 ~ Py < en < bpy ~ phen < NO2– < PR3 < CH3 < CN– ~ CO
From I- To H2O are weak field ligands.
From NCS– To CO are strong field ligands.
It is based on the interaction of metal with ligand. Greater the interaction of the ligand with metal, greater will be the splitting of d-orbitals and thus, strong pairing will take place. It is an experimentally determined series based on the absorption of light by complexes with different ligands.
Question 33.
Attempt any five of the following:
Give an example of the reaction:
(a) Cyanohydrin
(b) Acetal
(c) Semicarbazone
(d) Aldol
(e) Hemiacetal
(f) Oxime
(g) Ketal
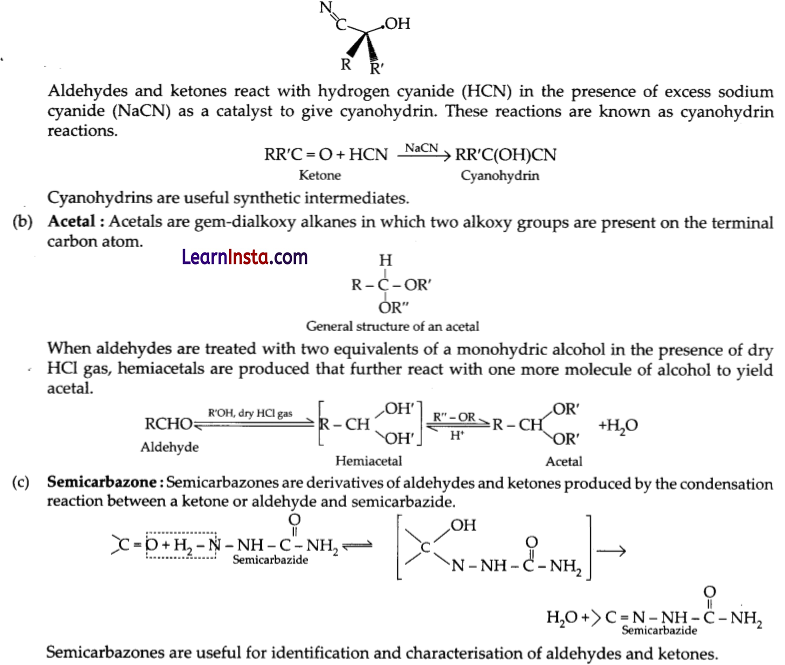
Answer:
(a) Cyanohydrin: Cyanohydrins are organic compounds having hydroxyl (- OH) and cyano (CN) groups on the same carbon atom