Students can access the CBSE Sample Papers for Class 12 Chemistry with Solutions and marking scheme Set 2 will help students in understanding the difficulty level of the exam.
CBSE Sample Papers for Class 12 Chemistry Set 2 with Solutions
Time Allowed: 3 hours
Maximum Marks: 70
General Instructions :
1. There are 33 questions in this question paper with internal choice
2. SECTION A consists of 16 multiple-choice questions carrying 1 mark each.
3. SECTION A consists of 16 multiple-choice questions carrying 1 mark each.
4. SECTION C consists of 7 short answer questions carrying 3 marks each.
5. SECTION D consists of 2 case-based questions carrying 4 marks each.
6. SECTION E consists of 3 long answer questions carrying 5 marks each.
7. All questions are compulsory.
8. Use of log tables and calculators is not allowed.
SECTION – A (16 Marks)
The following questions are multiple-choice questions with one correct answer. Each question carries 1 mark. There is no internal choice in this section.
Question 1.
The standard emf of a galvanic cell involving cell reaction with n = 2 is formed to be 0.295 V at 25° C. The equilibrium constant of the reaction would be:
(a) 1.0 × 1010
(b) 2.0 × 1011
(c) 4.0 × 1012
(d) 1.0 × 102
[Given F = 96500 (mol-1); R = 8.314 JK-1 mol-1]
Answer:
(a) 1.0 × 1010
Explanation: Given:
F = 96500 C mol-1
R = 8.314 JK-1 mol-1
T = 25°C = 25 + 273 = 298 K
E°cell = 0.295 V
To Find: Equilibrium constant = ?
By Nemst equation, Ecell = E°cell – \( \frac{2.303 \mathrm{RT}}{n \mathrm{~F}}\) log10K
At Equilibrium, Ecell = 0

Question 2.
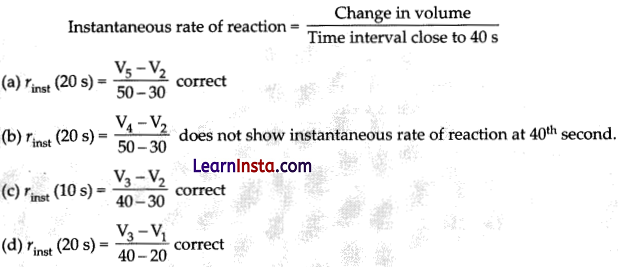
Consider the graph given in figure. Which of the following option does not show instantaneous rate of reaction at 40th s?
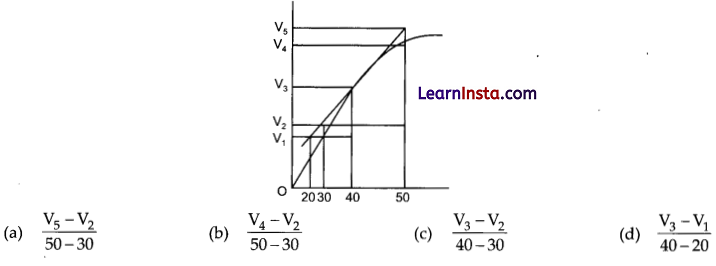
Answer:
(b) \(\frac{V_4-V_2}{50-30}\)
Explanation: Reaction occurring at smallest time interval is known as instantaneous rate of reaction at 40th s is rate of reaction during a small interval of time close to 40th s. Volume change during a small time interval close to 40 s i.e., 40 – 30 s, 50 – 40 s, 50 – 30 s, 40 – 20 s.
Question 3.
Which of the following is not a neutral ligand?
(a) H2O
(b) NH3
(c) ONO
(d) CO2
Answer:
(c) ONO
Explanation: Neutral ligands are those ligands that do not have any charge over them. Example- H2O, NH3, CO, C2H4, ONO– has charge on it, therefore, it is not neutral ligand.
![]()
Question 4.
Match the columns:
| Column I | Column II |
| A. Mass percentage | (p). Medicine and pharmacy |
| B Mass by volume | (q). Concentration of pollutants in water |
| C.. ppm | (r). Industrial chemical application |
| D. Volume percentage | (s). Liquid solutions |
(a) A-(q),B-(p),C-(s),D-(r)
(b) A-(r), B-(p), C-(q), D-(s)
(c) A-(r), B-(q), C-(s), D-(p)
(d) A-(s), B-(r), C-(p), D-(q)
Answer:
(b) A-(r), B-(p), C-(q), D-(s)
Explanation: Mass percentage is industrial chemical application, Mass by volume is medicine and pharmacy, ppm is concentration of pollutants in water, and volume percentage is liquid solutions.
Question 5.
The action of sodium on alkyl halide to form an alkane is called :
(a) Grignard reaction
(b) Wurtz coupling reaction
(c) Isocyanide reaction
(d) Halogenation reaction
Answer:
(b) Wurtz coupling reaction
Explanation: Wurtz-reaction is a coupling reaction in organic chemistry, where two alkyl halides
react with sodium metal in dry ether solution to form a higher alkane:
Question 6.
All galvanic cells do not contain :
(a) A cathode
(b) an anode
(c) ions
(d) a porous plate
Answer:
(d) a porous plate
Explanation: All galvanic cells do not contain a porous plate.
Question 7.
Maltose on hydrolysis gives:
(a) α-D-glucose
(b) a and β-D-glucose
(c) Glucose and fructose
(d) Fructose only
Answer:
(a) α-D-glucose
Explanation: Maltose is also known as malt sugar. It is a disaccharide, made up of two D-glucose units. The two units of glucose are linked with an alpha 1,4 glycosidic bond. Thus, maltose on hydrolysis gives 2 moles of α-D-glucose.
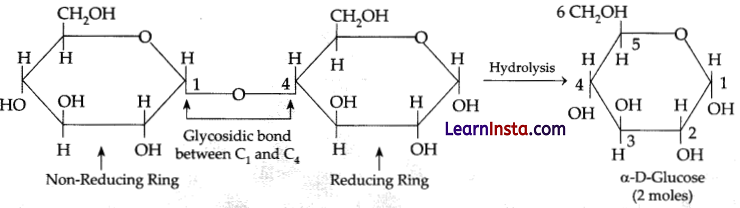
Question 8.
Identify ‘X’ in the reaction given below:
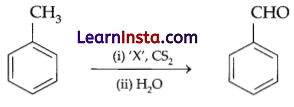
(a) CrO3
(b) CrO2Cl2
(c) Alkaline KMnO4
(d) Anhydrous AlCl3
Answer:
(b) CrO2Cl2
Explanation: This is known as Etard reaction. The chromyl chloride oxidises the methyl group to a chromium complex in CS2. This complex on hydrolysis gives benzaldehyde. For the given reaction, when toluene is treated with chromyl chloride in CS2, brown chromium complex is formed, which on hydrolysis gives benzaldehyde.
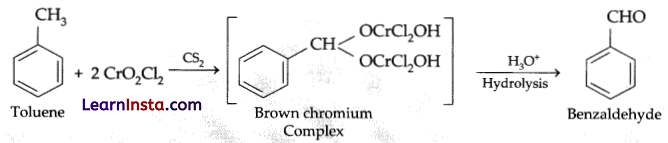
Question 9.
In the hydroboration-oxidation reaction of propene with diborane, H2O2 and NaOH, the organic compound formed is:
(a) CH3CH2OH
(b) CH3CH(OH)CH3
(c) CH3CH2CH2OH
(d) (CH3)3COH
Answer:
(c) CH3CH2CH2OH
Explanation: The hydroboration oxidation reaction converts an alkene into neutral alcohol by the addition of water across the double bond.
The hydrogen and hydroxyl groups are added in a syn addition leading to cis stereochemistry. Hydroboration oxidation is an anti-Markovnikov reaction, with the hydroxyl group attaching to the less-substituted carbon. In the hydroboration-oxidation reaction of propene the organic compound
formed is CH3CH2CH2OH.
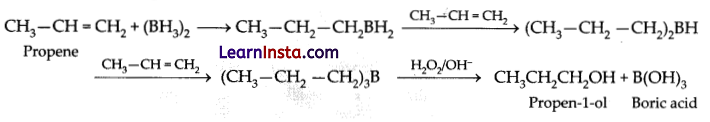
Question 10.
The linkage which holds various amino acid units in primary structures of proteins is :
(a) Glycoside linkage
(b) Peptide linkage
(c) Ionic linkage
(d) Hydrogen bond
Answer:
(b) Peptide linkage
Explanation: Peptide linkage is also known as peptide bond. It is an amide formed between -COOH and – NH2 group by elimination of a water molecule.
![]()
Question 11.
Benzoyl Chloride on reduction with H2/Pd-BaSO4 produces:
(a) Benzoic acid
(b) Benzyl alcohol
(c) Benzoyl sulphate
(d) Benzaldehyde
Answer:
(d) Benzaldehyde (Aldehyde, Ketone and carboxylic acid)
Explanation: A Rosenmund catalyst is used to reduce acyl chlorides to their corresponding aldehydes and is typically composed of palladium supported on BaSO4.
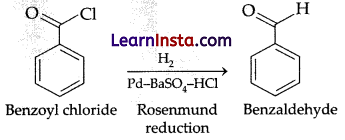
Question 12.
According to Werner’s theory of coordination compounds:
(a) Primary valency is ionisable
(b) Secondary valency is ionisable
(c) Primary and secondary valencies are ionisable
(d) Neither primary nor secondary valency is ionisable
Answer:
(a) Primary valency is ionisable
Explanation: Metals possess two types of valencies: primary (principal) or ionisable valency and secondary or non-ionisable valency.
Primary valencies: are those which a metal usually exhibits in the formation of its simple salts. Thus, in the formation of PtCl4, CuSO4, and AgCl. The primary valencies of Pt, Cu, and Ag are 4, 2 and 1, respectively. Primary valencies are satisfied by negative ions.
Secondary valencies: are those which a metal cation exercises towards a neutral molecule or negative group in forming its complex ions. Thus, secondary valencies may be satisfied by negative ions, neutral molecules having lone electron pair (Example: H2O, NH3 etc.) or even sometimes by some positive groups.
Question No.13 to 16 consist of two statements- Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true.
Question 13.
Assertion (A): During reduction of nitrobenzene, Fe + HCl is preferred as the reagent.
Reason (R): During oxidation of nitroalkanes into alkanamines the preferred reagent is Fe + HCl.
Answer:
(c) A is true, but R is false
Explanation: During the reduction of a nitro alkane, iron scrap (Fe) + HCl is preferred because, FeCl2 is produced which gets easily hydrolysed to form FeCl3 and releases FiCl during the reaction. The process, therefore, becomes economical as only a small amount of HC1 is required to initiate the reaction. Similarly, nitro alkanes can also be reduced with metals in acidic medium to the corresponding alkanamines. Thus, assertion is false but reason is true.
Question 14.
Assertion (A): (CH3)3-CONa and CH3CH2Br react to form (CH3)3C-O-CH2CH3
Reason (R): Good yields of ethers are obtained when ferf-alkyl halides are treated with alkoxides.
Answer:
(c) A is true, but R is false.
Explanation: (CH3)3CONa and CH3CH2Br react to form (CH3)3C-O-CH2CH3. Good yields of ether are obtained when primary alkyl halides are treated with alkoxides derived from any alcohol, 1°, 2°, or 3°.
Question 15.
Assertion (A): Transition metals and their many compounds act as good catalyst.
Reason (R): It is due to variable magnetic property.
Answer:
(c) A is true, but R is false.
Explanation: Transition metals and their many compounds act as good catalyst because they have variable valencies and show multiple oxidation states and transition metals sometime form Unstable intermediate complex which provide a new path with lower activation energy for the reaction. Also, transition elements provide a suitable surface for the reaction to take place. Thus, assertion is true but reason is false.
![]()
Question 16.
Assertion (A): Phenol and alcohol can be distinguished by NaOH.
Reason (R): Alcohol is a very weak acid as compared to Phenol.
Answer:
(b) Both A and R are true but R is the not the not the correct explanation of A.
Explanation: Phenol and alcohol can be distinguished by NaOH. Both alcohols and phenol are weak acid, the alcohols are less acidic than phenol because it is very tough to remove ‘H’ ion from alcohol. Phenol can lose ion easily because phenoxide ion formed is stabilized to some extent. This is as the negative charge on the oxygen atom is delocalised around the ring. Thus, both assertion and reason are true but reason is not the correct explanation of assertion.
SECTION – B (10 Marks)
This section contains 5 questions with internal choice in one questions. The following questions are very short answer type and carry 2 marks each.
Question 17.
(a) Electrical conductance through metals is called the metallic or electrical conductance. What are the factors on which electrical conductance depends upon?
(b) Classify the following as primary or secondary types of batteries: Lead storage battery, Leclanche cell, Ni-Cd cell, Mercury cell.
Answer:
(a) Electrical conductance is due to the movement of electrons in metals. It depends upon the following :
1. The nature and structure of the metal.
2. The number of valence electrons per atom.
3. Temperature (it decreases with increase of temperature).
(b) Primary battery: Leclanche cell and mercury cell.
Secondary battery: Lead storage battery and Ni-Cd cell.
Question 18.
A solution is prepared by dissolving 43.0 g of naphthalene (Mm = 128.0 g mol-1) in 117.0 g of benzene (Mm = 78.0 g mol-1). Calculate the mole fractions of the two components of the solution.
Answer:
Number of moles n = m/mm where m is the mass and mm is the molar mass.
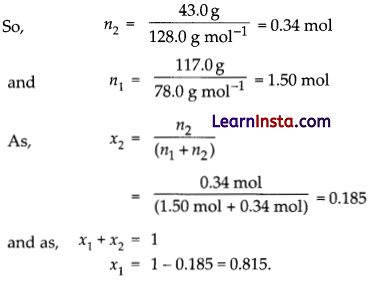
Question 19.
Answer the following:
(a) What is Fehling’s solution?
(b) To what oxidation state ethanal converts Fehling’s solution.
Answer:
(a) Fehling’s solution is alkaline solution of CuSO4 along with some Rochelle salt.
(b) Ethanal converts Cu(II) of Fehling’s solution to Cu(I) i.e., +1 state.
Question 20.
What are the factors on which conductivity of an electrolyte depend?
Answer:
The conductivity of an electrolyte depends upon:
1. The nature of electrolyte
2. Size of the ions produced
3. Nature of solvent and its viscosity.
4. Concentration of electrolyte.
5. Temperature
![]()
Question 21.
(a) Which pyrimidine base is present in RNA?
(b) What are nucleotides?
OR
Give the following reactions:
(a) Reaction of glucose with HI.
(b) Reaction of glucose with acetic anhydride.
Answer:
(a) Uracil is the pyrimidine base present in RNA.
(b) Nucleotide is a unit which consists of a phosphate group, a pentose sugar and an aromatic heterocyclic nitrogenous base.
OR
(a) Glucose on prolonged heating with the HI gives the M-hexane which has 6 carbon atoms connected with each other in the straight chain. Thus, this concludes that all the 6 carbon atoms in the glucose molecule are also connected linearly in the form of a straight chain.
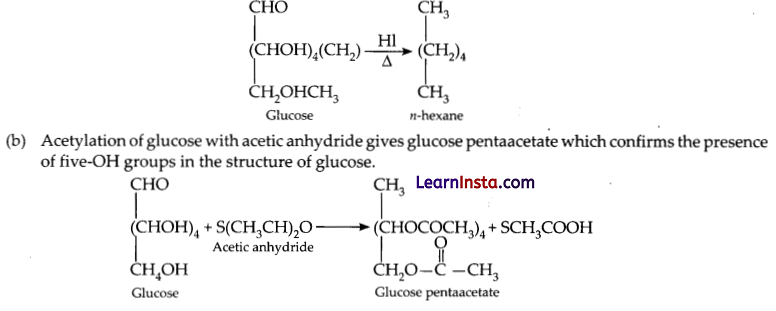
SECTION – C (21 Marks)
This section contains 7 questions with internal choice in one questions. The following questions are short answer type and carry 3 marks each.
Question 22.
Name two water soluble vitamins, their sources and the diseases caused due to their deficiency in diet.
Answer:
| Vitamin | Sources | Deficiency disease |
| 1. Vitamin B2 (Riboflavin or Lactoflavin) |
Milk, yeast, green vegetables, meat, liver, kidney, egg white etc. Daily dosage is 2-3 mg. | Retards growth, causes inflammation of tongue (glossitis), dermatitis and cheilosis (cracking or Assuring) at comers of mouth and lips. |
| 2. Vitamin C (Ascorbic acid) | Citms fruits, green leafy vegetables, chillies, sprouted pulses and germinated grains. Daily dosage is 75 gm | Scurvy (bleeding of gums), pyorrhea (loosening and bleeding of teeth). |
Question 23.
Write the Nemst equation. Calculate EMF of the following cell at 25°C.
Pt(s) I Br2(l) I Br (0.010 M) I I H+ (0.030 M) I H2(g) (1 bar) I Pt(s)
Given: E°Br2/Br = 1-08V
Answer:
The cell reaction is:
2Br–(aq) + 2H+(aq) -> Br2(I) + H2(g)
Using Nemst equation and substituting the values.
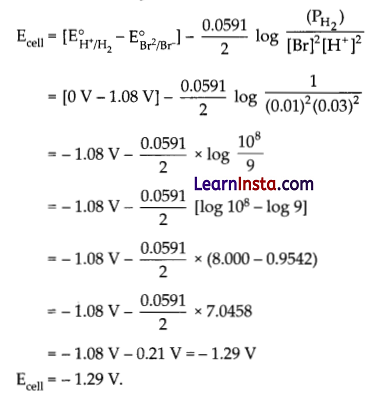
Question 24.
Attempt any two.
(a) How would you obtain acetophenone from phenol?
(b) Solubility of alcohols in water decreases with increasing size of alkyl group, why?
(c) What is Reimer-Tiemann reaction? Give equation for that.
Answer:
(a) Acetophenone can be obtained from phenol by the reaction of phenol with zinc dust in the presence of heat.
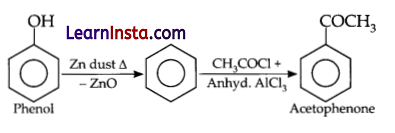
(b) Solubility of alkyl or aryl group in water depends upon the following factors:
1. Hydrogen bonding: Greater the extent of H-bonding, higher is the solubility.
2. Size of the alkyl/aryl group: Higher the hydrocarbon part, lower is the extent of H-bonding and hence lower is the solubility.
Thus, the solubility of alkyl or aryl group decreases with increase in size.
(c) Phenols react with CHCl3 and alkali to give salicylaldehyde. This Reaction is known as reimer tiemann reaction.
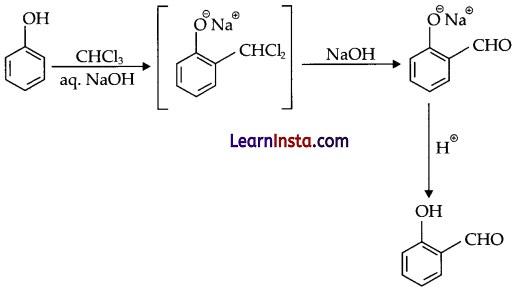
Question 25.
(a) A significant increase in the density may be noted from titanium (atomic no. 22) to copper (atomic no. 29), give reason.
(b) The transition elements form a large number of complex compounds, why?
(c) Draw the structure of dichromate ion.
Answer:
(a) On moving from Ti to Cu, the atomic radii decreases due to increase in nuclear charge. The decrease in metallic radius from Ti (Z = 22) to copper (Z = 29) coupled with increase in atomic mass results in a general increase in the density of these elements..
(b) The transition elements form a large number of complex compounds due to the comparatively smaller sizes of the metal ions, their high ionic charges and the availability of d-orbitals for bond formation.
(c) The structure of dichromate ion is as follows:
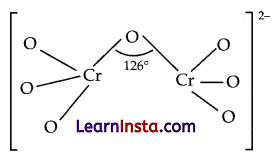
Question 26.
Following ions are given:
Cr2+, Cu2+, Cu+, Fe2+, Fe3+, Mn3+
Identify the ion which is:
(a) A strong reducing agent.
(b) Unstable in aqueous solution.
(c) A strong oxiding agent.
Give suitable reason in each.
Answer:
(a) Cr2+ is a strong reducing agent as it is very easily oxidized to more stable Cr3+ (d3) where it attains a stable half-filled t32g configuration.
(b) Cu+ is unstable in aqueous solution because it disproportionates in water to form Cu2+ and Cu.
(c) Mn3+ is a strong oxidising agent because it gets reduced to Mn2+ (d5) in the process and attains an extra stable half-filled d orbital configuration.
![]()
Question 27.
The change in concentration of a reactant or product in unit time is called rate or reaction. The reaction:
2 NO(g) + O2(g) → 2NO2(g) was studied by the initial rate method. The following kinetic data was obtained.
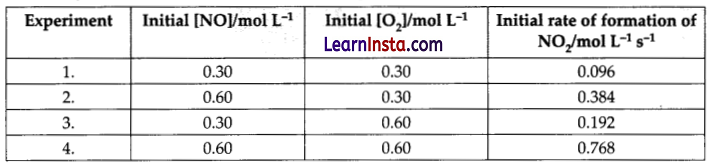
(a) What do you understand by rate law?
(b) What is the order of the reaction with respect to NO and O2?
(c) Determine the rate law for the reaction.
Answer:
(a) Rate law is an experimentally determined expression which relates the rate of reaction with concentration of the reactants.
(b) Suppose the order of reaction w.r.t. NO is x and w.r.t. O2, it is y, then
Rate = k [NO]x [O2]y
0.096 = k (0.30)x (0.30)y …(i)
0.384 = k (0.60)x (0.30)y …(ii)
0.192 = k (0.30)x (0.60)y …(iii)
0.768 = k (0.60)x (0.60)y …(iv)
Dividing equation (ii) by (i), we get
\( \frac{0.384}{0.096}=\frac{k(0.60)^x(0.30)^y}{k(0.30)^x(0.30)^y}\)
4 = 2x or 22 = 2x or x = 2
Dividing (iv) by (ii), we get
\( \frac{0.768}{0.384}=\frac{k(0.60)^x(0.60)^y}{k(0.60)^x(0.30)^y}\)
4 = 2y or y = 1
Hence, order of reaction w.r.t. NO = 2
order of reaction w.r.t. O2 =1
(c) Rate = k [NO]2 [O2]1
Question 28.
The decomposition of phosphine PHy proceeds according to the following equation: 4PH3(g) →P4(g) + 6H2(g)
Its found that the reaction follows the following rate equation:
Rate = k[PH3]
The half life period of PH3 is 37.9s at 120°C.
(i) How much time is required for 3/4th of the PH3 to decompose?
(ii) What fraction of the original sample of PH3 remains behind after 1 minute?
Answer:
(i) As we can see from the rate equation that its a first order reaction.
Initial concentration, let be = a
Then concentration after time, t = 3/4 a = x
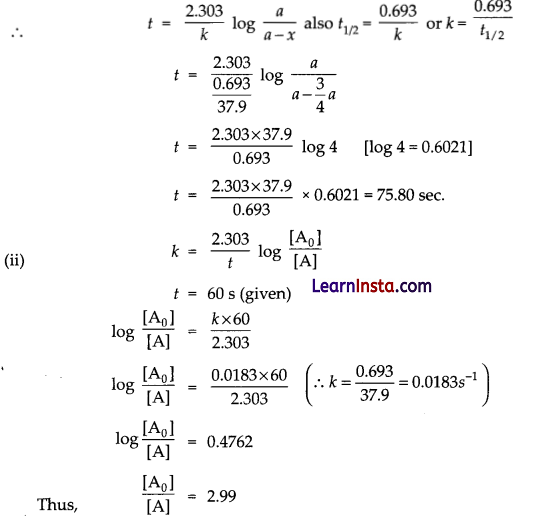
SECTION – D (8 Marks)
The following questions are case-based questions. Each question has an internal choice and carries 4 (1+1+2) marks each. Read the passage carefully and answer the questions that follow.
Question 29.
Any substituent which can increase stability of conjugate base of carboxylic acid i.e., carboxylate ion will increase its acidity and a substituent decreasing the stability of carboxylate ion will decrease the acidity of carboxylic acid. It means electron withdrawing group will increase and electron donating group will decrease the acidity of carboxylic acid. Carboxylic acids are more acidic than phenols as carboxylate ion is stabilized by two equivalent resonating structures in which negative charge is at the more electronegative oxygen atom. In comparison although phenoxide ion is also resonance stabilized but resonating structures and are non-equivalent in which negative charge is at less electronegative carbon atom, hence resonating structures of phenoxide are less important than that of carboxylate ions.
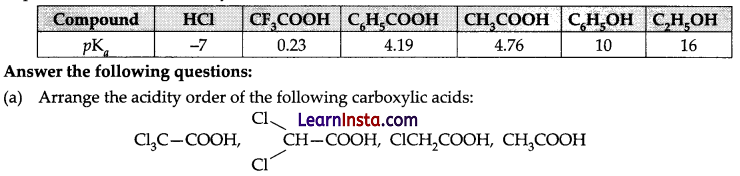
(b) Why formic acid is more acidic than acetic acid?
(c) Explain the acidity order of the following acids:
(i) FCH2COOH, ClCH2COOH, BrCH2COOH, ICH2COOH
(ii) HC- C-COOH, CH2 = CH-COOH, CH3COOH
OR
Why vinyl or allyl carboxylic acids are highly acidic?
Answer:
(a) With the increase in the number of halogen atoms the strength of acid also increases. Hence, the acidity order will be as follows:

(b) Formic acid is more acidic than acetic acid because of the following reasons:
1. +I-effect of –CH3 group distabilises the acetate ion (CH3COO–)
2. Due to the small size of formate ion , it can be easily solvated.
(c) 1. Greater the electron with drawing power of halogen atom, stronger will be the acid.
Thus the acidity order is:
FCH2COOH > ClCH2COOH > BrCH2COOH > ICH2COOH
2. With increase in the ‘s’-character of the carbon atom electronegativity will increase.
Electronegativity order is – sp > sp2 > sp3 hybridised carbon, ‘s’-character (50%) (33.3%) (25%) Hence, the acidity order will be as follows:
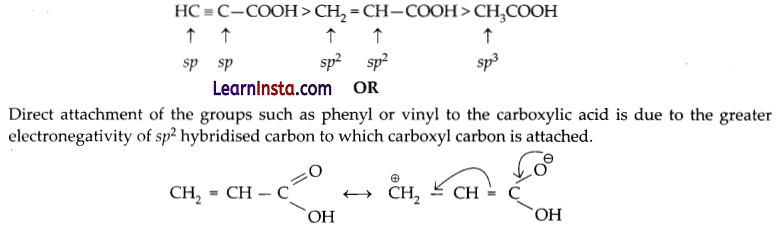
![]()
Question 30.
The elevation in boiling point on addition of a non-volatile, non-electrolyte and solid solute to a solvent can be easily described graphically. The vapour pressure of the pure solvent or solution increases with increase in temperature. The variation of vapour pressure of solvent and solution at different temperature is shown in figure below:
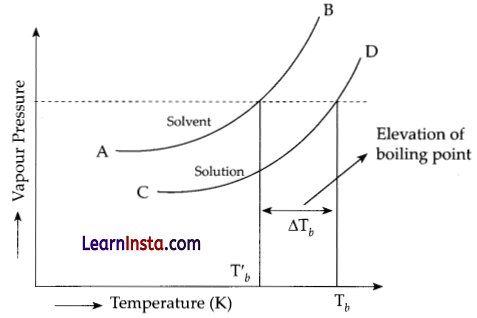
The curve AB gives the vapour pressure of the pure solvent and the curve CD gives the vapour pressure for the solution at different temperatures. The curve CD lies below the curve AB which corresponds to the vapour pressure of the pure solvent. This is due to the fact that the vapour pressure of the solution is less than that of the pure solvent at all temperatures.
Answer the following questions:
(a) Do you think that boiling point is a colligative property?
(b) Why does the use of pressure cooker reduces cooking time?
(c) Distinguish between the boiling point of a liquid and the normal boiling point of a liquid.
OR
5 g each of two solutes A and B (Molar mass of A > B) are dissolved separately in 50 g each of the same solvent. Which will show greater elevation of boiling point?
Answer:
(a) Elevation in boiling point is a colligative property but boiling point is not a colligative property.
(b) At higher pressure over the liquid, the liquid boils at higher temperature. That is why, cooking occurs faster.
(c) The boiling point is the temperature at which the vapour pressure of the liquid equals to the atmospheric pressure. The normal boiling point is the temperature at which the vapour pressure is 1 atom.
OR
Solution containing solute ‘B’ will show greater elevation in boiling point as ΔTb α \( \frac{1}{\mathrm{M}}\)
SECTION – E (15 Marks)
The following questions are long answer type and carry 5 marks each. All questions have an internal choice.
Question 31.
Attempt any five of the following:
Write the structure of the major organic product in each of the following reactions:
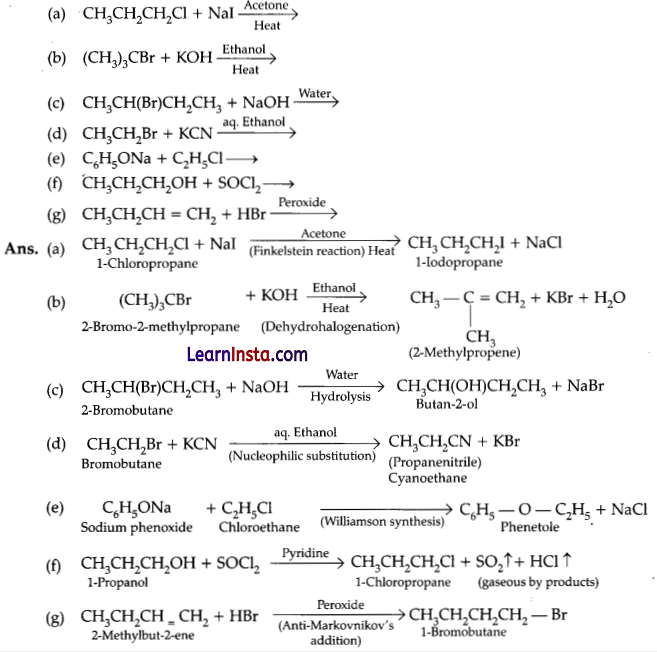
Question 32.
Account for the following:
(a) [Cr(NH3)6]3+ is a paramagnetic while [Ni(CN4)]2- is diamagnetic. Explain why?
(b) [NiCl4]2- is paramagnetic while [Ni(CO)4] is diamagnetic though both are tetrahedral.
(c) [Fe(H2O)6]3+ is strongly paramagnetic where as [Fe(CN)6]3- is weakly paramagnetic.
OR
(a) What is crystal field splitting energy? Flow does the magnitude of Δ0 decide the actual configuration of d-orbitals in a coordination entity?
(b) Discuss briefly giving an example in each case the role of coordination compounds in:
(i) Biological system
(ii) Medicinal chemistry
(iii) Analytical chemistry
(iv) Extraction or metallurgy of metals
Answer:
(a) Cr is in the +3 oxidation state i.e., d3 configuration. Also NH3 is a weak field ligand that does not cause the pairing of the electrons in the 3d-orbital.
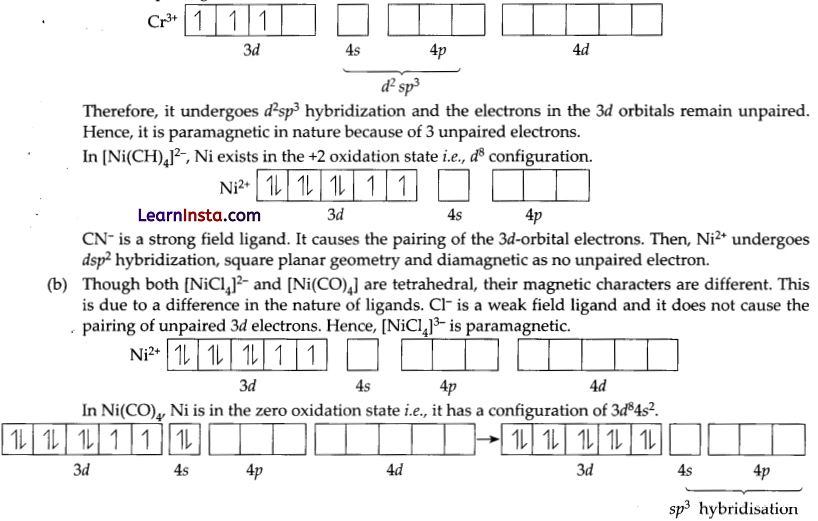
But CO is a strong field ligand. Therefore, it causes the pairing of unpaired 3d electrons. Also, it causes the 4s electrons to shift to the 3d-orbital, thereby giving rise to sp3 hybridization. Since, no unpaired electrons are present in this case, [Ni(CO)4] is diamagnetic.
(c) In. both [Fe(H2O6]3+ and [Fe(CN)6]3- . Fe exists in the +3 oxidation state i.e., in d5 configuration.
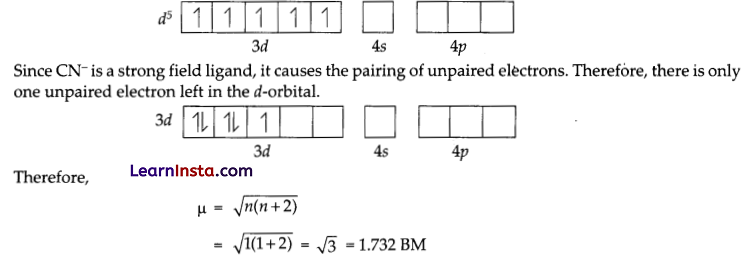
On the other hand, H2O is a weak field ligand. Therefore, it cannot cause the pairing of electrons. This means that the number of unpaired electrons is 5.
Therefore, \(\begin{aligned}
\mu & =\sqrt{n(n+2)} \\
& =\sqrt{5(5+2)}=\sqrt{35}=5.916 \mathrm{BM}
\end{aligned}\)
Hence, [Fe(H2O6]3+ is strongly paramagnetic, while [Fe(CN)6]3- is weakly paramagnetic.
OR
(a) The degenerate d-orbitals (in a spherical field environment) split into two levels i.e., eg and t2g in the presence of ligands. The spliting of the degenerate levels due to the presence of ligands is called the crystal-field splitting while the energy difference between the two levels eg (high) and t2g (low)] is called the crystal-field splitting energy. It is denoted by Δ0 for octahedral complexes.
After the orbitals have split, the filling of the electrons takes place. According to Hund’s rule 1 electron is been filled in the three t2g1 orbitals, the filling of the fourth electron takes place in two ways. It can enter the eg orbital (giving rise to t2g 3e1g0 electronic configuration) or the pairing of the electrons can take place in the t2g orbitals (giving rise to t2g1 4eg0 electronic configuration). If the Δ0 value of a ligand is less than the pairing energy (P) (Δ0 < p) then the electrons enter the eg orbital. On the other hand, if the Δ0 value of a ligand is more than the pairing energy (P) (Δ0 < p) then the electrons enter the t2g orbital. Thus, the value of A0 decides the configuration and magnetic matrix of the complex.
- (b) (i) Role of coordination compounds in biological system: Photosynthesis is made possible by the presence of the chlorophyll pigment. This pigment is a coordination compound of magnesium. In the human biological system, several coordination compounds play an important role. For example, the oxygen-carrier of blood, i.e., haemoglobin, is a coordination compound of iron. Enzymes sometimes use Chelation to perform biological catalytic reactions.
- (ii) Role of coordination compounds in medicinal chemistry: Certain coordination compounds of platinum (for example, cis-platin) are used for inhibiting the growth of humours. Specially the cis-isomer. Now a days it is replaced by dinuclear platinum complex because of certain side effects.
- (iii) Role of coordination compounds in analytical chemistry: During salt analysis, a number of basic radicals are detected with the help of the colour changes they exhibit with different reagents. These colour changes are a result of the coordination compounds or complexes that the basic radicals form with different ligands, e.g., Cu2+ ion detection where deep blue and chocolate colour ppt. are produced where both are complex compounds.
- (iv) Role of coordination compounds in extraction or metallurgy of metals: The process of extraction of some of the metals from their ores involves the formation of complexes. For examples, in aqueous solution, gold combines with cyanide ions to form [Au(CN)2]. From this solution, gold is later extracted by the addition of zinc metal. Also silver ore can be extracted using same method.
![]()
Question 33.
A compound [A] has molecular formula as C3H7NO. It gives the following reactions:
(a) Hydrolysis of [A] gives an amine [B] and carboxylic acid [C].
(b) Amine [B] with Hinsberg’s reagent forms water insoluble product. Identify [A], [B] and [C]. Justify
your answer.
(c) Acid [C] on treatment with Tollen’s reagent gives a positive silver mirror test.
OR
(a) Account for the following:
(i) Aniline dissolves in hydrochloric acid.
(ii) Sulphanilic acid is insoluble in water but soluble in aqueous base and aqueous mineral acids.
(b) Arrange the following:
(i) In decreasing order of the pKb values: C2H5NH2, C2H5NHCH3 (C2H5)2NH and C6H5NH2.
(ii) In increasing order of basic strength: C6H5NH2, C6H5N(CH3)2, (C2H5)NH and CH3NH2.
(iii) In increasing order of basic strength:
1. Aniline, p-nitroaniline and p-toluidine
2. C6H5NH2, C6H5NHCH3, C6H5CH2NH2.
Answer:
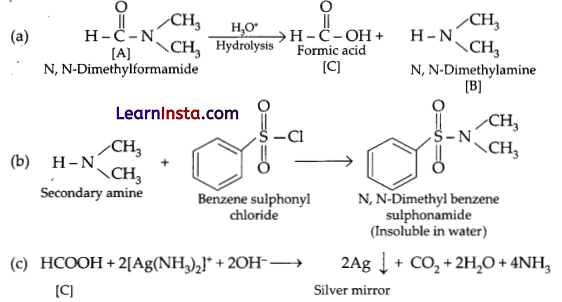
Compound [A] C3H7NO, must be an amide because it gives carboxylic acid and an amine on hydrolysis.
\( \mathrm{C}_3 \mathrm{H}_7 \mathrm{NO} \xrightarrow{\mathrm{H}_3 \mathrm{O}^{+}} \text {Acid }[C]+ \text { Amine }[B]\)
Amine [B] must be a secondary amine as it produces aninsoluble compound on treatment with Hinsberg’s reagent, i.e., benzene sulphonyl chloride. Also as only formic acid gives a positive silver mirror test, hence acid [C] must be HCOOH. Keeping in mind the above facts, the only structure of compound [A] that supports the above is:
OR
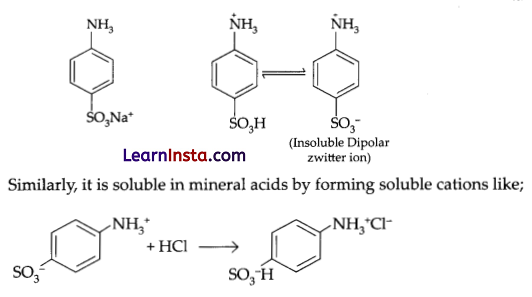
(a) (i) Aniline being a base dissolves in acid. The lone pair of aniline nitrogen (- NH2) forms a bond with H+ of hydrochloric acid to give water soluble anilinium ion C6H5+NH3.
(ii) Sulphanilic acid forms Zwitter ion and hence not soluble in water and organic solvents. But, it is soluble in base [NaOH] by forming soluble sulphonate ion
(b) (i) Higher pKb value, lower is the basic strength. In C2H5NH2, only one -C2H5 group is present while in (C2H5)2NH, two -C2H5 groups are present. Thus, the +I effect is more in (C2H5)2NH than in C2H5NH2. Therefore, the electron density over the N-atom is more in (C2H5)2NH than in C2H5NH2. Hence, (C2H5)2NH is more basic than C2H5NH2.
C6H5NHCH3 is more basic than C6H5NH2 and less basic than (C2H5)2NH and C2H5NH2 due to the delocalization of the lone pair in the former two. Hence, the order of increasing basicity of the given compounds is as follows:
C6H5NH2 < C6H5NHCH3 < C2H5NH2 < (C2H5)2NH
Therefore, decreasing pKb values are:
C6H5NH2 > C6H5NHCH3 > C2H5NH2 > (C2H5)2NH
(ii) C6H5N(CH3)2 is more basic than C6H5NH2 due to presence of +1 effect of two -CH3 groups in C6H5N(CH3)2. Further, CH3NH2 contains one -CH3 group while (C2H5)2NH contains two -C2H5 groups. Thus, (C2H5)2NH is more basic than C2H5NH2.
Now, C6H5N(CH3)2 is less basic than CH3NH2 because of the -I effect of -C6H5 group. Hence, the decreasing order of the basic strengths of the given compounds is as follows: (C2H5)2NH > CH3NH2 > C6H5N(CH3)2 > C6H5NH2.
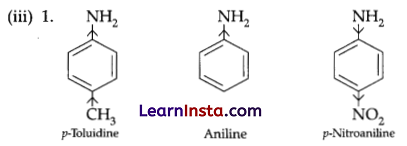
In p-toluidine, the presence of electron-donating -CH3 group increases the electron density on the N-atom. Thus, p-toluidine is more basic than aniline.
On the other hand, the presence of electron withdrawing –NO2 group decreases the electron density over the N-atom in p-nitroaniline. Thus, p-nitroaniline is less basic than aniline. Hence, the increasing order of the basic strengths of the given compounds is as follows:
p-Nitroaniline < Aniline < p-Toluidine.
2. C6H5NHCH3 is more basic than C6H5NH2 due to the presence of electron-donating -CH3 group in C6H5NHCH3. Again, in C6H5NHCH3 -C6H5 group is directly attached to the N-atom. However, it is not so in C6H5CH2NH2. Thus, in C6H5NHCH3 the – I effect of -C6H5 group decreases the electron density over the N-atom. Therefore, C6H5CH2NH2 is more basic than C6H5NHCH3. Hence, the increasing order of the basic strengths of the given compounds is as follows:
C6H5NH2 < C6H5NHCH3 < C6H5CH2NH2